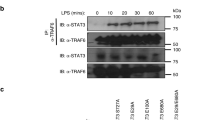
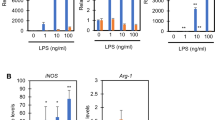
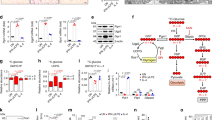
Abstract
Macrophages activated by the Gram-negative bacterial product lipopolysaccharide switch their core metabolism from oxidative phosphorylation to glycolysis1. Here we show that inhibition of glycolysis with 2-deoxyglucose suppresses lipopolysaccharide-induced interleukin-1β but not tumour-necrosis factor-α in mouse macrophages. A comprehensive metabolic map of lipopolysaccharide-activated macrophages shows upregulation of glycolytic and downregulation of mitochondrial genes, which correlates directly with the expression profiles of altered metabolites. Lipopolysaccharide strongly increases the levels of the tricarboxylic-acid cycle intermediate succinate. Glutamine-dependent anerplerosis is the principal source of succinate, although the ‘GABA (γ-aminobutyric acid) shunt’ pathway also has a role. Lipopolysaccharide-induced succinate stabilizes hypoxia-inducible factor-1α, an effect that is inhibited by 2-deoxyglucose, with interleukin-1β as an important target. Lipopolysaccharide also increases succinylation of several proteins. We therefore identify succinate as a metabolite in innate immune signalling, which enhances interleukin-1β production during inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
10 April 2013
The product code for the anti-HIF-1α antibody was corrected.
References
Rodriguez-Prados, J. C. et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J. Immunol. 185, 605–614 (2010)
Krawczyk, C. M. et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115, 4742–4749 (2010)
Pan, H. & Wu, X. Hypoxia attenuates inflammatory mediators production induced by Acanthamoeba via Toll-like receptor 4 signaling in human corneal epithelial cells. Biochem. Biophys. Res. Commun. 420, 685–691 (2012)
Hiscott, J. et al. Characterization of a functional NF-κB site in the human interleukin 1β promoter: evidence for a positive autoregulatory loop. Mol. Cell. Biol. 13, 6231–6240 (1993)
Ghisletti, S. et al. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARγ. Mol. Cell 25, 57–70 (2007)
Zhang, W. et al. Evidence that hypoxia-inducible factor-1 (HIF-1) mediates transcriptional activation of interleukin-1β (IL-1β) in astrocyte cultures. J. Neuroimmunol. 174, 63–73 (2006)
Peyssonnaux, C. et al. Cutting edge: essential role of hypoxia inducible factor-1α in development of lipopolysaccharide-induced sepsis. J. Immunol. 178, 7516–7519 (2007)
Chandel, N. S. et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 275, 25130–25138 (2000)
Sumbayev, V. V. LPS-induced Toll-like receptor 4 signalling triggers cross-talk of apoptosis signal-regulating kinase 1 (ASK1) and HIF-1α protein. FEBS Lett. 582, 319–326 (2008)
Selak, M. A. et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 7, 77–85 (2005)
Koivunen, P. et al. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J. Biol. Chem. 282, 4524–4532 (2007)
Rubic, T. et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nature Immunol. 9, 1261–1269 (2008)
Walmsley, S. R. et al. Prolyl hydroxylase 3 (PHD3) is essential for hypoxic regulation of neutrophilic inflammation in humans and mice. J. Clin. Invest. 121, 1053–1063 (2011)
Du, J. et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334, 806–809 (2011)
Yan, Y., Dalmasso, G., Sitaraman, S. & Merlin, D. Characterization of the human intestinal CD98 promoter and its regulation by interferon-γ. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G535–G545 (2007)
Choi, S. & Silverman, R. B. Inactivation and inhibition of γ-aminobutyric acid aminotransferase by conformationally restricted vigabatrin analogues. J. Med. Chem. 45, 4531–4539 (2002)
Walker, S. D. & Kalviainen, R. Non-vision adverse events with vigabatrin therapy. Acta Neurol. Scand. (suppl.)124, 72–82 (2011)
Bossy-Wetzel, E., Newmeyer, D. D. & Green, D. R. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 17, 37–49 (1998)
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010)
Rotstein, O. D., Pruett, T. L. & Simmons, R. L. Lethal microbial synergism in intra-abdominal infections. Escherichia coli and Bacteroides fragilis. Arch. Surg. 120, 146–151 (1985)
Toma, I. et al. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J. Clin. Invest. 118, 2526–2534 (2008)
Sadagopan, N. et al. Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am. J. Hypertens. 20, 1209–1215 (2007)
Gimenez-Roqueplo, A. P. et al. The R22X mutation of the SDHD gene in hereditary paraganglioma abolishes the enzymatic activity of complex II in the mitochondrial respiratory chain and activates the hypoxia pathway. Am. J. Hum. Genet. 69, 1186–1197 (2001)
Dahia, P. L. et al. A HIF1α regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 1, 72–80 (2005)
Hobert, J. A., Mester, J. L., Moline, J. & Eng, C. Elevated plasma succinate in PTEN, SDHB, and SDHD mutation-positive individuals. Genet. Med. 14, 616–619 (2012)
Pistollato, F. et al. Hypoxia and succinate antagonize 2-deoxyglucose effects on glioblastoma. Biochem. Pharmacol. 80, 1517–1527 (2010)
Masters, S. L. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nature Immunol. 11, 897–904 (2010)
Doyle, S. L. et al. The GOLD domain-containing protein TMED7 inhibits TLR4 signalling from the endosome upon LPS stimulation. Nature Commun. 3, 707 (2012)
West, A. P. et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 (2011)
MacKenzie, E. D. et al. Cell-permeating α-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol. Cell. Biol. 27, 3282–3289 (2007)
Acknowledgements
We thank the European Research Council, Science Foundation Ireland, the Health Research Board, European Union FP7 programme ‘TIMER’, Wellcome Trust, National Institutes of Health, Helmsley Trust, Nestle Research Centre, VESKI, The Duquesne University Hunkele Dreaded Disease Award, The Interleukin Foundation and the National Health and Medical Research Council for funding. We also thank R. Thompson for assistance with Hif1a−/− mice and M. Murphy for discussions.
Author information
Authors and Affiliations
Contributions
G.M.T. designed and did experiments, analysed data and wrote the paper; L.A.J.O. conceived ideas and oversaw the research programme; A.M.C, E.M.P., A.F.M. and J.A. designed and did experiments and analysed data; C.F., N.J.B., B.K., N.H.F., L.Z., A.G., Z.T., S.S.J., S.C.C., S.W., K.P. and F.C.B did experiments; G.G., R.J.X., C.C., M.H. and B.E.C. performed bioinformatic analysis; E.C., V.N., M.W., C.T.T., H.L., S.L.M., E.G., V.K. and C.C., provided advice and reagents; P.E.A. and R.J.X. conceived ideas and oversaw a portion of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-26. (PDF 684 kb)
Supplementary Data
This file contains Supplementary Table 1. (XLSX 79 kb)
Rights and permissions
About this article
Cite this article
Tannahill, G., Curtis, A., Adamik, J. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013). https://doi.org/10.1038/nature11986
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11986