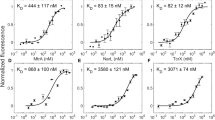
Abstract
Bacterial pathogens have evolved specific effector proteins that, by interfacing with host kinase signalling pathways, provide a mechanism to evade immune responses during infection1,2. Although these effectors contribute to pathogen virulence, we realized that they might also serve as valuable synthetic biology reagents for engineering cellular behaviour. Here we exploit two effector proteins, the Shigella flexneri OspF protein3 and Yersinia pestis YopH protein4, to rewire kinase-mediated responses systematically both in yeast and mammalian immune cells. Bacterial effector proteins can be directed to inhibit specific mitogen-activated protein kinase pathways selectively in yeast by artificially targeting them to pathway-specific complexes. Moreover, we show that unique properties of the effectors generate new pathway behaviours: OspF, which irreversibly inactivates mitogen-activated protein kinases4, was used to construct a synthetic feedback circuit that shows novel frequency-dependent input filtering. Finally, we show that effectors can be used in T cells, either as feedback modulators to tune the T-cell response amplitude precisely, or as an inducible pause switch that can temporarily disable T-cell activation. These studies demonstrate how pathogens could provide a rich toolkit of parts to engineer cells for therapeutic or biotechnological applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ribet, D. & Cossart, P. Pathogen-mediated posttranslational modifications: a re-emerging field. Cell 143, 694–702 (2010)
Broberg, C. A. & Orth, K. Tipping the balance by manipulating post-translational modifications. Curr. Opin. Microbiol. 13, 34–40 (2010)
Zhang, Z. Y. et al. Expression, purification, and physicochemical characterization of a recombinant Yersinia protein tyrosine phosphatase. J. Biol. Chem. 267, 23759–23766 (1992)
Li, H. et al. The phosphothreonine lyase activity of a bacterial type III effector family. Science 315, 1000–1003 (2007)
Shaw, A. S. & Filbert, E. L. Scaffold proteins and immune-cell signalling. Nature Rev. Immunol. 9, 47–56 (2009)
Dong, C., Davis, R. J. & Flavell, R. A. MAP kinases in the immune response. Annu. Rev. Immunol. 20, 55–72 (2002)
Khalil, A. S. & Collins, J. J. Synthetic biology: applications come of age. Nature Rev. Genet. 11, 367–379 (2010)
Purnick, P. E. & Weiss, R. The second wave of synthetic biology: from modules to systems. Nature Rev. Mol. Cell Biol. 10, 410–422 (2009)
Bashor, C. J., Helman, N. C., Yan, S. & Lim, W. A. Using engineered scaffold interactions to reshape MAP kinase pathway signaling dynamics. Science 319, 1539–1543 (2008)
Kramer, R. W. et al. Yeast functional genomic screens lead to identification of a role for a bacterial effector in innate immunity regulation. PLoS Pathog. 3, e21 (2007)
Zhu, Y. et al. Structural insights into the enzymatic mechanism of the pathogenic MAPK phosphothreonine lyase. Mol. Cell 28, 899–913 (2007)
Nelson, D. E. et al. Oscillations in NF-κB signaling control the dynamics of gene expression. Science 306, 704–708 (2004)
Cai, L., Dalal, C. K. & Elowitz, M. B. Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature 455, 485–490 (2008)
Muzzey, D., Gomez-Uribe, C. A., Mettetal, J. T. & van Oudenaarden, A. A systems-level analysis of perfect adaptation in yeast osmoregulation. Cell 138, 160–171 (2009)
Mettetal, J. T., Muzzey, D., Gomez-Uribe, C. & van Oudenaarden, A. The frequency dependence of osmo-adaptation in Saccharomyces cerevisiae. Science 319, 482–484 (2008)
Hersen, P., McClean, M. N., Mahadevan, L. & Ramanathan, S. Signal processing by the HOG MAP kinase pathway. Proc. Natl Acad. Sci. USA 105, 7165–7170 (2008)
Pelet, S. et al. Transient activation of the HOG MAPK pathway regulates bimodal gene expression. Science 332, 732–735 (2011)
Morgan, R. A., Dudley, M. E. & Rosenberg, S. A. Adoptive cell therapy: genetic modification to redirect effector cell specificity. Cancer J. 16, 336–341 (2010)
June, C. H., Blazar, B. R. & Riley, J. L. Engineering lymphocyte subsets: tools, trials and tribulations. Nature Rev. Immunol. 9, 704–716 (2009)
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 (2010)
Brentjens, R., Yeh, R., Bernal, Y., Riviere, I. & Sadelain, M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol. Ther. 18, 666–668 (2010)
Arbibe, L. et al. An injected bacterial effector targets chromatin access for transcription factor NF-κB to alter transcription of host genes involved in immune responses. Nature Immunol. 8, 47–56 (2007)
Gerke, C., Falkow, S. & Chien, Y. H. The adaptor molecules LAT and SLP-76 are specifically targeted by Yersinia to inhibit T cell activation. J. Exp. Med. 201, 361–371 (2005)
Lin, J. & Weiss, A. The tyrosine phosphatase CD148 is excluded from the immunologic synapse and down-regulates prolonged T cell signaling. J. Cell Biol. 162, 673–682 (2003)
Yao, T., Mecsas, J., Healy, J. I., Falkow, S. & Chien, Y. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, yopH. J. Exp. Med. 190, 1343–1350 (1999)
Kieback, E., Charo, J., Sommermeyer, D., Blankenstein, T. & Uckert, W. A safeguard eliminates T cell receptor gene-modified autoreactive T cells after adoptive transfer. Proc. Natl Acad. Sci. USA 105, 623–628 (2008)
de Witte, M. A. et al. An inducible caspase 9 safety switch can halt cell therapy-induced autoimmune disease. J. Immunol. 180, 6365–6373 (2008)
Bonini, C. et al. The suicide gene therapy challenge: how to improve a successful gene therapy approach. Mol. Ther. 15, 1248–1252 (2007)
Ciceri, F. et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I–II study. Lancet Oncol. 10, 489–500 (2009)
Brandman, O. et al. Feedback loops shape cellular signals in space and time. Science 322, 390–395 (2008)
Peisajovich, S. G., Garbarino, J. E., Wei, P. & Lim, W. A. Rapid diversification of cell signaling phenotypes by modular domain recombination. Science 328, 368–372 (2010)
Lee, P. J., Helman, N. C., Lim, W. A. & Hung, P. J. A microfluidic system for dynamic yeast cell imaging. Biotechniques 44, 91–95 (2008)
Acknowledgements
We thank K. Orth for the YopH plasmid; K. McNally and S. Neou for tissue culture support; H. El-Samad, C. Voigt, C. Tang and the Lim laboratory for discussions. We acknowledge the 2007 UCSF iGEM team (M. Chen, E. Chou, J. Huang, L. Jann, E. Meltzer, A. Ng and R. Ovadia) for their initial work on bacterial effectors in yeast. This work was supported by American Cancer Society fellowship PF-09-137-01-TBE (W.W.W), a Li Foundation Fellowship (P.W.), a California Institute for Regenerative Medicine fellowship (grant number TG2-01153) (J.S.P.), National Institutes of Health grants PN2EY016546, RO1GM055040, RO1GM062583 and P50GM081879 (W.A.L.), the NSF Synthetic Biology and Engineering Research Center (W.A.L.), the Packard Foundation (W.A.L.) and the Howard Hughes Medical Institute (A.W. and W.A.L.).
Author information
Authors and Affiliations
Contributions
P.W., S.G.P. and W.A.L. initiated the project in yeast. W.W.W., E.E.C., A.W. and W.A.L. initiated the project in T cells. P.W., W.W.W. and W.A.L. wrote the paper. P.W. and S.G.P. planned and performed the experiments in yeast. W.W.W., P.W., E.E.C., J.J.O. and J.S.P. planned and performed the experiment in T cells.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Tables 1-8, Supplementary Figures 1-9 and additional references. (PDF 1112 kb)
Rights and permissions
About this article
Cite this article
Wei, P., Wong, W., Park, J. et al. Bacterial virulence proteins as tools to rewire kinase pathways in yeast and immune cells. Nature 488, 384–388 (2012). https://doi.org/10.1038/nature11259
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11259