Abstract
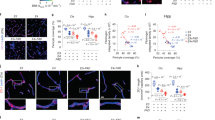
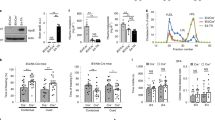
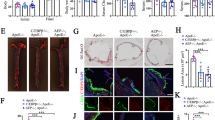
Human apolipoprotein E has three isoforms: APOE2, APOE3 and APOE41. APOE4 is a major genetic risk factor for Alzheimer’s disease2,3 and is associated with Down’s syndrome dementia and poor neurological outcome after traumatic brain injury and haemorrhage3. Neurovascular dysfunction is present in normal APOE4 carriers4,5,6 and individuals with APOE4-associated disorders3,7,8,9,10. In mice, lack of Apoe leads to blood–brain barrier (BBB) breakdown11,12, whereas APOE4 increases BBB susceptibility to injury13. How APOE genotype affects brain microcirculation remains elusive. Using different APOE transgenic mice, including mice with ablation and/or inhibition of cyclophilin A (CypA), here we show that expression of APOE4 and lack of murine Apoe, but not APOE2 and APOE3, leads to BBB breakdown by activating a proinflammatory CypA–nuclear factor-κB–matrix-metalloproteinase-9 pathway in pericytes. This, in turn, leads to neuronal uptake of multiple blood-derived neurotoxic proteins, and microvascular and cerebral blood flow reductions. We show that the vascular defects in Apoe-deficient and APOE4-expressing mice precede neuronal dysfunction and can initiate neurodegenerative changes. Astrocyte-secreted APOE3, but not APOE4, suppressed the CypA–nuclear factor-κB–matrix-metalloproteinase-9 pathway in pericytes through a lipoprotein receptor. Our data suggest that CypA is a key target for treating APOE4-mediated neurovascular injury and the resulting neuronal dysfunction and degeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
05 May 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41586-023-06118-0
References
Mahley, R. W., Weisgraber, K. H. & Huang, Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J. Lipid Res. 50 (Suppl.). S183–S188 (2009)
Kim, J., Basak, J. M. & Holtzman, D. M. The role of apolipoprotein E in Alzheimer’s disease. Neuron 63, 287–303 (2009)
Verghese, P. B., Castellano, J. M. & Holtzman, D. M. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 10, 241–252 (2011)
Thambisetty, M., Beason-Held, L., An, Y., Kraut, M. A. & Resnick, S. M. APOE ε4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch. Neurol. 67, 93–98 (2010)
Sheline, Y. I. et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J. Neurosci. 30, 17035–17040 (2010)
Reiman, E. M. et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc. Natl Acad. Sci. USA 101, 284–289 (2004)
Zlokovic, B. V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature Rev. Neurosci. 12, 723–738 (2011)
Snowdon, D. A. et al. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. J. Am. Med. Assoc. 277, 813–817 (1997)
Vermeer, S. E. et al. Silent brain infarcts and the risk of dementia and cognitive decline. N. Engl. J. Med. 348, 1215–1222 (2003)
Ruitenberg, A. et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann. Neurol. 57, 789–794 (2005)
Methia, N. et al. ApoE deficiency compromises the blood–brain barrier especially after injury. Mol. Med. 7, 810–815 (2001)
Hafezi-Moghadam, A., Thomas, K. L. & Wagner, D. D. ApoE deficiency leads to a progressive age-dependent blood–brain barrier leakage. Am. J. Physiol. Cell Physiol. 292, C1256–C1262 (2007)
Nishitsuji, K., Hosono, T., Nakamura, T., Bu, G. & Michikawa, M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood–brain barrier model. J. Biochem. 286, 17536–17542 (2011)
Bell, R. D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010)
Armulik, A. et al. Pericytes regulate the blood–brain barrier. Nature 468, 557–561 (2010)
Sullivan, P. M. et al. Reduced levels of human apoE4 protein in an animal model of cognitive impairment. Neurobiol. Aging 32, 791–801 (2011)
Satoh, K. et al. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nature Med. 15, 649–656 (2009)
Jin, Z. G. et al. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler. Thromb. Vasc. Biol. 24, 1186–1191 (2004)
Handschumacher, R. E., Harding, M. W., Rice, J., Drugge, R. J. & Speicher, D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science 226, 544–547 (1984)
Begley, D. J. et al. Permeability of the blood–brain barrier to the immunosuppressive cyclic peptide cyclosporin A. J. Neurochem. 55, 1222–1230 (1990)
Grammas, P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J. Neuroinflammation 8, 26 (2011)
Paul, J., Strickland, S. & Melchor, J. P. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer’s disease. J. Exp. Med. 204, 1999–2008 (2007)
Zhong, Z. et al. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J. Clin. Invest. 119, 3437–3449 (2009)
Candelario-Jalil, E. et al. Matrix metalloproteinases are associated with increased blood–brain barrier opening in vascular cognitive impairment. Stroke 42, 1345–1350 (2011)
Garcia-Alloza, M. et al. Matrix metalloproteinase inhibition reduces oxidative stress associated with cerebral amyloid angiopathy in vivo in transgenic mice. J. Neurochem. 109, 1636–1647 (2009)
Jaeger, L. B. et al. Testing the neurovascular hypothesis of Alzheimer’s disease: LRP-1 antisense reduces blood–brain barrier clearance, increases brain levels of amyloid-β protein, and impairs cognition. J. Alzheimers Dis. 17, 553–570 (2009)
Yang, Y., Lu, N., Zhou, J., Chen, Z. N. & Zhu, P. Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. Rheumatology 47, 1299–1310 (2008)
Deane, R. et al. apoE isoform-specific disruption of amyloid β peptide clearance from mouse brain. J. Clin. Invest. 118, 4002–4013 (2008)
Masliah, E. et al. Neurodegeneration and cognitive impairment in apoE-deficient mice is ameliorated by infusion of recombinant apoE. Brain Res. 751, 307–314 (1997)
Zipser, B. D. et al. Microvascular injury and blood–brain barrier leakage in Alzheimer’s disease. Neurobiol. Aging 28, 977–986 (2007)
Acknowledgements
We would like to thank the National Institute of Health for grants R37NS34467 (B.V.Z.); R37AG23084 (B.V.Z.); RO1AG039452 (B.V.Z.); and R37AG13956 (D.M.H.) used to support this study.
Author information
Authors and Affiliations
Contributions
R.D.B. designed and performed experiments, analysed data and contributed to writing the paper. E.A.W. designed and performed experiments. I.S. performed experiments. A.P.S. performed CBF experiments. R.D. designed experiments and analysed data. Z.W. gathered pilot data. D.M.H. provided guidance and edited the paper. C.B. designed experiments and edited the paper. A.A. performed pilot cadaverine studies. J.S. generated pilot data. B.C.B. provided guidance and edited the paper. B.V.Z. designed experiments, analysed data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-15, Supplementary Materials and Methods, Supplementary Discussion, Supplementary Tables 1-3 and Supplementary References. (PDF 1515 kb)
Rights and permissions
About this article
Cite this article
Bell, R., Winkler, E., Singh, I. et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 (2012). https://doi.org/10.1038/nature11087
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11087