Abstract
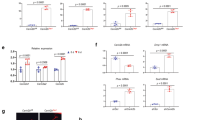
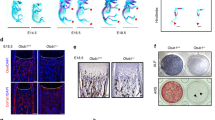
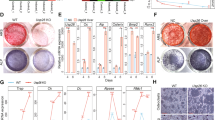
The bony skeleton is maintained by local factors that regulate bone-forming osteoblasts and bone-resorbing osteoclasts, in addition to hormonal activity. Osteoprotegerin protects bone by inhibiting osteoclastic bone resorption, but no factor has yet been identified as a local determinant of bone mass that regulates both osteoclasts and osteoblasts. Here we show that semaphorin 3A (Sema3A) exerts an osteoprotective effect by both suppressing osteoclastic bone resorption and increasing osteoblastic bone formation. The binding of Sema3A to neuropilin-1 (Nrp1) inhibited receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclast differentiation by inhibiting the immunoreceptor tyrosine-based activation motif (ITAM) and RhoA signalling pathways. In addition, Sema3A and Nrp1 binding stimulated osteoblast and inhibited adipocyte differentiation through the canonical Wnt/β-catenin signalling pathway. The osteopenic phenotype in Sema3a−/− mice was recapitulated by mice in which the Sema3A-binding site of Nrp1 had been genetically disrupted. Intravenous Sema3A administration in mice increased bone volume and expedited bone regeneration. Thus, Sema3A is a promising new therapeutic agent in bone and joint diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Takayanagi, H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nature Rev. Immunol. 7, 292–304 (2007)
Elefteriou, F. Regulation of bone remodeling by the central and peripheral nervous system. Arch. Biochem. Biophys. 473, 231–236 (2008)
Seeman, E. & Delmas, P. D. Bone quality–the material and structural basis of bone strength and fragility. N. Engl. J. Med. 354, 2250–2261 (2006)
Teitelbaum, S. L. & Ross, F. P. Genetic regulation of osteoclast development and function. Nature Rev. Genet. 4, 638–649 (2003)
Martin, T. J. & Sims, N. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol. Med. 11, 76–81 (2005)
Lewiecki, E. M. New targets for intervention in the treatment of postmenopausal osteoporosis. Nature Rev. Rheumatol. 7, 631–638 (2011)
Rachner, T. D., Khosla, S. & Hofbauer, L. C. Osteoporosis: now and the future. Lancet 377, 1276–1287 (2011)
Reid, I. R. et al. Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J. Bone Miner. Res. 25, 2256–2265 (2010)
Odvina, C. V. et al. Severely suppressed bone turnover: a potential complication of alendronate therapy. J. Clin. Endocrinol. Metab. 90, 1294–1301 (2005)
Suda, T. et al. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 20, 345–357 (1999)
Nakashima, T. et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nature Med. 17, 1231–1234 (2011)
Xiong, J. et al. Matrix-embedded cells control osteoclast formation. Nature Med. 17, 1235–1241 (2011)
Simonet, W. S. et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89, 309–319 (1997)
Luo, Y., Raible, D. & Raper, J. A. Collapsin: A protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 75, 217–227 (1993)
Tran, T. S., Kolodkin, A. L. & Bharadwaj, R. Semaphorin regulation of cellular morphology. Annu. Rev. Cell Dev. Biol. 23, 263–292 (2007)
Negishi-Koga, T. et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nature Med. 17, 1473–1480 (2011)
Takegahara, N. et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nature Cell Biol. 8, 615–622 (2006)
Matsuo, K. & Irie, N. Osteoclast-osteoblast communication. Arch. Biochem. Biophys. 473, 201–209 (2008)
Taniguchi, M. et al. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron 19, 519–530 (1997)
Gomez, C. et al. Expression of Semaphorin-3A and its receptors in endochondral ossification: potential role in skeletal development and innervation. Dev. Dyn. 234, 393–403 (2005)
Behar, O., Golden, J. A., Mashimo, H., Schoen, F. J. & Fishman, M. C. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 383, 525–528 (1996)
Jacquin, C., Gran, D. E., Lee, S. K., Lorenzo, J. A. & Aguila, H. L. Identification of multiple osteoclast precursor populations in murine bone marrow. J. Bone Miner. Res. 21, 67–77 (2006)
Neufeld, G. & Kessler, O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nature Rev. Cancer 8, 632–645 (2008)
Gu, C. et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 5, 45–57 (2003)
Ashburner, B. P., Westerheide, S. D. & Baldwin, A. S., Jr The p65 (RelA) subunit of NF-κB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol. Cell. Biol. 21, 7065–7077 (2001)
Takayanagi, H. et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 3, 889–901 (2002)
Koga, T. et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428, 758–763 (2004)
Takahashi, T. & Strittmatter, S. M. Plexina1 autoinhibition by the plexin sema domain. Neuron 29, 429–439 (2001)
Narazaki, M. & Tosato, G. Ligand-induced internalization selects use of common receptor neuropilin-1 by VEGF165 and semaphorin3A. Blood 107, 3892–3901 (2006)
Nishikawa, K. et al. Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J. Clin. Invest. 120, 3455–3465 (2010)
Gimble, J. M., Zvonic, S., Floyd, Z. E., Kassem, M. & Nuttall, M. E. Playing with bone and fat. J. Cell. Biochem. 98, 251–266 (2006)
Krishnan, V., Bryant, H. U. & Macdougald, O. A. Regulation of bone mass by Wnt signaling. J. Clin. Invest. 116, 1202–1209 (2006)
Takada, I., Kouzmenko, A. P. & Kato, S. Wnt and PPARγ signaling in osteoblastogenesis and adipogenesis. Nature Rev. Rheumatol. 5, 442–447 (2009)
Toyofuku, T. et al. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nature Neurosci. 8, 1712–1719 (2005)
Wu, X. et al. Rac1 activation controls nuclear localization of β-catenin during canonical Wnt signaling. Cell 133, 340–353 (2008)
Takegahara, N. et al. Integral roles of a guanine nucleotide exchange factor, FARP2, in osteoclast podosome rearrangements. FASEB J. 24, 4782–4792 (2010)
Nagashima, M. et al. Bisphosphonate (YM529) delays the repair of cortical bone defect after drill-hole injury by reducing terminal differentiation of osteoblasts in the mouse femur. Bone 36, 502–511 (2005)
Tang, Y. et al. TGF-β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nature Med. 15, 757–765 (2009)
Hayden, J. M., Mohan, S. & Baylink, D. J. The insulin-like growth factor system and the coupling of formation to resorption. Bone 17, S93–S98 (1995)
Kawai, M., Mödder, U. I., Khosla, S. & Rosen, C. J. Emerging therapeutic opportunities for skeletal restoration. Nature Rev. Drug Discov. 10, 141–156 (2011)
Grigoriadis, A. E. et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266, 443–448 (1994)
Asagiri, M. et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 202, 1261–1269 (2005)
Mizuno, A. et al. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem. Biophys. Res. Commun. 247, 610–615 (1998)
Kaifu, T. et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J. Clin. Invest. 111, 323–332 (2003)
Hayashi, M. et al. Ly49Q, an ITIM-bearing NK receptor, positively regulates osteoclast differentiation. Biochem. Biophys. Res. Commun. 393, 432–438 (2010)
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005)
Acknowledgements
We are grateful to D. D. Ginty and A. L. Kolodkin for providing the Nrp1Sema− knockin mice. We thank Y. Goshima for providing vectors and technical help. We thank A. Yamaguchi, H. Asahara and F. Suto for providing reagents and technical help. We also thank K. Okamoto, T. Negishi-Koga, K. Nishikawa, H. Inoue, T. Suda, T. Ando, Y. Kunisawa, Y. Ogihara and S. Fukuse for discussion and assistance. This work was supported in part by a grant for the Exploratory Research for Advanced Technology Program, the Takayanagi Osteonetwork Project from the Japan Science and Technology Agency; Grant-in-Aid for Young Scientist A from the Japan Society for the Promotion of Science (JSPS); a Grant-in-Aid for Challenging Exploratory Research from the JSPS; grants for the Global Center of Excellence Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and grants from the Tokyo Biochemical Research Foundation, the Life Science Foundation of Japan, Takeda Science Foundation, Uehara Memorial Foundation, Naito Foundation, BMKK RA Research Fund and Astellas Foundation for Research on Metabolic Disorders.
Author information
Authors and Affiliations
Contributions
M.H. performed most of the experiments, interpreted the results and prepared the manuscript. T.N. performed immunohistochemical experiments and provided advice on project planning and data interpretation and prepared the manuscript. M.T. provided technical help. T.K. conducted the GeneChip analysis. A.K. provided advice on project planning and technical help. H.T. directed, supervised the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-10 and Supplementary Table 1. (PDF 10755 kb)
Rights and permissions
About this article
Cite this article
Hayashi, M., Nakashima, T., Taniguchi, M. et al. Osteoprotection by semaphorin 3A. Nature 485, 69–74 (2012). https://doi.org/10.1038/nature11000
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11000
This article is cited by
-
Bone-targeting engineered small extracellular vesicles carrying anti-miR-6359-CGGGAGC prevent valproic acid-induced bone loss
Signal Transduction and Targeted Therapy (2024)
-
Treadmill exercise with nanoselenium supplementation affects the expression of Irisin/FNDC5 and semaphorin 3A in rats exposed to cigarette smoke extract
3 Biotech (2024)
-
Sema4D as a biomarker for Predicting rheumatoid arthritis disease activity
Clinical Rheumatology (2024)
-
Wnt16 Increases Bone-to-Implant Contact in an Osteopenic Rat Model by Increasing Proliferation and Regulating the Differentiation of Bone Marrow Stromal Cells
Annals of Biomedical Engineering (2024)
-
Pathophysiological functions of semaphorins in the sympathetic nervous system
Inflammation and Regeneration (2023)