Abstract
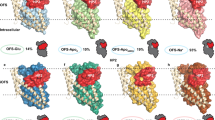
Neurotransmitter sodium symporters are integral membrane proteins that remove chemical transmitters from the synapse and terminate neurotransmission mediated by serotonin, dopamine, noradrenaline, glycine and GABA (γ-aminobutyric acid). Crystal structures of the bacterial homologue, LeuT, in substrate-bound outward-occluded and competitive inhibitor-bound outward-facing states have advanced our mechanistic understanding of neurotransmitter sodium symporters but have left fundamental questions unanswered. Here we report crystal structures of LeuT mutants in complexes with conformation-specific antibody fragments in the outward-open and inward-open states. In the absence of substrate but in the presence of sodium the transporter is outward-open, illustrating how the binding of substrate closes the extracellular gate through local conformational changes: hinge-bending movements of the extracellular halves of transmembrane domains 1, 2 and 6, together with translation of extracellular loop 4. The inward-open conformation, by contrast, involves large-scale conformational changes, including a reorientation of transmembrane domains 1, 2, 5, 6 and 7, a marked hinge bending of transmembrane domain 1a and occlusion of the extracellular vestibule by extracellular loop 4. These changes close the extracellular gate, open an intracellular vestibule, and largely disrupt the two sodium sites, thus providing a mechanism by which ions and substrate are released to the cytoplasm. The new structures establish a structural framework for the mechanism of neurotransmitter sodium symporters and their modulation by therapeutic and illicit substances.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Hertting, G. & Axelrod, J. Fate of tritiated noradrenaline at the sympathetic nerve-endings. Nature 192, 172–173 (1961)
Nelson, N. The family of Na+/Cl− neurotransmitter transporters. J. Neurochem. 71, 1785–1803 (1998)
Saier, M. H. J. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 64, 354–411 (2000)
Hahn, M. K. & Blakely, R. D. Monoamine transporter gene structure and polymorphisms in relation to psychiatric and other complex disorders. Pharmacogenomics J. 2, 217–235 (2002)
Klimek, V. et al. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J. Neurosci. 17, 8451–8458 (1997)
Richerson, G. B. & Wu, Y. Role of the GABA transporter in epilepsy. Adv. Exp. Med. Biol. 548, 76–91 (2004)
Amara, S. G. & Sonders, M. S. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depend. 51, 87–96 (1998)
Mitchell, P. A general theory of membrane transport from studies of bacteria. Nature 180, 134–136 (1957)
Jardetzky, O. Simple allosteric model for membrane pumps. Nature 211, 969–970 (1966)
Yamashita, A., Singh, S. K., Kawate, T., Jin, Y. & Gouaux, E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature 437, 215–223 (2005)
Singh, S., Yamashita, A. & Gouaux, E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature 448, 952–956 (2007)
Singh, S. K., Piscitelli, C. L., Yamashita, A. & Gouaux, E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science 322, 1655–1661 (2008)
Zhou, Z. et al. LeuT-desipramine structure reveals how antidepressants block neurotransmitter uptake. Science 317, 1390–1393 (2007)
Zhou, Z. et al. Antidepressant specificity of serotonin transporter suggested by three LeuT–SSRI structures. Nature Struct. Mol. Biol. 16, 652–657 (2009)
Weyand, S. et al. Structure and molecular mechanism of a nucleobase–cation–symport-1 family transporter. Science 322, 709–713 (2008)
Faham, S. et al. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science 321, 810–814 (2008)
Shaffer, P. L., Goehring, A., Shankaranarayanan, A. & Gouaux, E. Structure and mechanism of a Na+-independent amino acid transporter. Science 325, 1010–1014 (2009)
Shimamura, T. et al. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science 328, 470–473 (2010)
Krishnamurthy, H., Piscitelli, C. L. & Gouaux, E. Unlocking the molecular secrets of sodium-coupled transporters. Nature 459, 347–355 (2009)
Shaikh, S. A. & Tajkhorshid, E. Modeling and dynamics of the inward-facing state of a Na+/Cl− dependent neurotransmitter transporter homologue. PLOS Comput. Biol. 6, e1000905 (2010)
Claxton, D. P. et al. Ion/substrate-dependent conformational dynamics of a bacterial homolog of neurotransmitter:sodium symporters. Nature Struct. Mol. Biol. 17, 822–829 (2010)
Forrest, L. R. et al. Mechanism for alternating access in neurotransmitter transporters. Proc. Natl Acad. Sci. USA 105, 10338–10343 (2008)
Zhao, Y. et al. Single-molecule dynamics of gating in a neurotransmitter transporter homologue. Nature 465, 188–193 (2010)
Boudker, O. & Verdon, G. Structural perspectives on secondary active transporters. Trends Pharmacol. Sci. 31, 418–426 (2010)
Piscitelli, C. L., Krishnamurthy, H. & Gouaux, E. Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site. Nature 468, 1129–1132 (2010)
Kawate, T. & Gouaux, E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 14, 673–681 (2006)
Kniazeff, J. et al. An intracellular interaction network regulates conformational transitions in the dopamine transporter. J. Biol. Chem. 283, 17691–17701 (2008)
Loland, C. J., Norregaard, L., Litman, T. & Gether, U. Generation of an activating Zn2+ switch in the dopamine transporter: mutation of an intracellular tyrosine constitutively alters the conformational equilibrium of the transport cycle. Proc. Natl Acad. Sci. USA 99, 1683–1688 (2002)
Celik, L., Schiott, B. & Tajkhorshid, E. Substrate binding and formation of an occluded state in the leucine transporter. Biophys. J. 94, 1600–1612 (2008)
Harding, M. M. Small revisions to predicted distances around metal sites in proteins. Acta Crystallogr. D 62, 678–682 (2006)
Zhang, Y.-W. & Rudnick, G. The cytoplasmic substrate permeation pathway of serotonin transporter. J. Biol. Chem. 281, 36213–36220 (2006)
Rudnick, G. The cytoplasmic permeation pathway of neurotransmitter transporters. Biochemistry 50, 7462–7475 (2011)
Ben-Yona, A. & Kanner, B. I. Transmembrane domain 8 of the γ-aminobutyric acid transporter GAT-1 lines a cytoplasmic accessibility pathway into its binding pocket. J. Biol. Chem. 284, 9727–9732 (2009)
Tao, Z., Zhang, Y. W., Agyiri, A. & Rudnick, G. Ligand effects on cross-linking support a conformational mechanism for serotonin transport. J. Biol. Chem. 284, 33807–33814 (2009)
Rosenberg, A. & Kanner, B. I. The substrates of the γ-aminobutyric acid transporter GAT-1 induce structural rearrangements around the interface of transmembrane domains 1 and 6. J. Biol. Chem. 283, 14376–14383 (2008)
Henry, L. K., Adkins, E. M., Han, Q. & Blakely, R. D. Serotonin and cocaine-sensitive inactivation of human serotonin transporters by methanethiosulfonates targeted to transmembrane domain I. J. Biol. Chem. 278, 37052–37063 (2003)
Zomot, E. & Kanner, B. I. The interaction of the γ-aminobutyric acid transporter GAT-1 with the neurotransmitter is selectively impaired by sulfhydryl modification of a conformationally sensitive cysteine residue engineered into extracellular loop IV. J. Biol. Chem. 278, 42950–42958 (2003)
Mitchell, S. M., Lee, E., Garcia, M. L. & Stephan, M. M. Structure and function of extracellular loop 4 of the serotonin transporter as revealed by cysteine-scanning mutagenesis. J. Biol. Chem. 279, 24089–24099 (2004)
Smicun, Y., Campbell, S. D., Chen, M. A., Gu, H. & Rudnick, G. The role of external loop regions in serotonin transport. Loop scanning mutagenesis of the serotonin transporter external domain. J. Biol. Chem. 274, 36058–36064 (1999)
Hirayama, B. A., Diez-Sampedro, A. & Wright, E. M. Common mechanisms of inhibition for the Na+/glucose (hSGLT1) and Na+/Cl−/GABA (hGAT1) cotransporters. Br. J. Pharmacol. 134, 484–495 (2001)
Jacobs, M. T., Zhang, Y. W., Campbell, S. D. & Rudnick, G. Ibogaine, a noncompetitive inhibitor of serotonin transport, acts by stabilizing the cytoplasm-facing state of the transporter. J. Biol. Chem. 282, 29441–29447 (2007)
Shi, L., Quick, M., Zhao, Y., Weinstein, H. & Javitch, J. A. The mechanism of a neurotransmitter:sodium symporter-inward release of Na+ and substrate is triggered by a substrate in a second binding site. Mol. Cell 30, 667–677 (2008)
Zhao, Y. et al. Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue. Nature 474, 109–113 (2011)
Pantanowitz, S., Bendahan, A. & Kanner, B. I. Only one of the charged amino acids located in the transmembrane α-helices of the γ-aminobutyric acid transporter (subtype A) is essential for its activity. J. Biol. Chem. 268, 3222–3225 (1993)
Bennett, E. R., Su, H. & Kanner, B. I. Mutation of arginine 44 of GAT-1, a (Na+ + Cl−)-coupled γ-aminobutyric acid transporter from rat brain, impairs net flux but not exchange. J. Biol. Chem. 275, 34106–34113 (2000)
Loland, C. J., Granas, C., Javitch, J. A. & Gether, U. Identification of intracellular residues in the dopamine transporter critical for regulation of transporter conformation and cocaine binding. J. Biol. Chem. 279, 3228–3238 (2004)
Chen, N., Rickey, J., Berfield, J. L. & Reith, M. E. Aspartate 345 of the dopamine transporter is critical for conformational changes in substrate translocation and cocaine binding. J. Biol. Chem. 279, 5508–5519 (2004)
Watanabe, A. et al. The mechanism of sodium and substrate release from the binding pocket of vSGLT. Nature 468, 988–991 (2010)
Quick, M. et al. State-dependent conformations of the translocation pathway in the tyrosine transporter Tyt1, a novel neurotransmitter:sodium symporter from Fusobacterium nucleatum . J. Biol. Chem. 281, 26444–26454 (2006)
Shi, L. & Weinstein, H. Conformational rearrangements to the intracellular open states of the LeuT and ApcT transporters are modulated by common mechanisms. Biophys. J. 99, L103–L105 (2010)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Collaborative Computational Project 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Matthews, B. W. Solvent content of protein crystals. J. Mol. Biol. 33, 491–497 (1968)
McCoy, A. J. Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr. D 63, 32–41 (2007)
Kopp, J. & Schwede, T. The SWISS-MODEL Repository: new features and functionalities. Nucleic Acids Res. 34, D315–D318 (2006)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D 58, 1948–1954 (2002)
Painter, J. & Merritt, E. A. Optimal description of a protein structure in terms of multiple groups undergoing TLS motion. Acta Crystallogr. D 62, 439–450 (2006)
Terwilliger, T. C. Using prime-and-switch phasing to reduce model bias in molecular replacement. Acta Crystallogr. D 60, 2144–2149 (2004)
Kelley, L. A. & Sternberg, M. J. Protein structure prediction on the Web: a case study using the Phyre server. Nature Protocols 4, 363–371 (2009)
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010)
Acknowledgements
We thank D. Cawley for monoclonal antibody production, L. Vaskalis for help with illustrations and the staff at the Advanced Photon Source beamline 24-ID-E and at the Advanced Light Source beamline 5.0.2 for their assistance with X-ray data collection and processing. We are grateful to E. Haddadian, T. Sosnick and K. Freed for assistance in refining the backbone torsional and side-chain angles using their unpublished TOP algorithm. We thank all Gouaux laboratory members, especially C. Piscitelli and S. K. Singh, for discussions and helpful suggestions throughout the project. This work was supported by the National Institutes of Health. E.G. is an investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
H.K. and E.G. contributed to all aspects of the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Tables 1-2, Supplementary Figures 1-13 with legends, a Supplementary Discussion and Supplementary Movie legend. (PDF 3978 kb)
Supplementary Movie 1
The movie depicts the conformational changes associated with isomerization from the open-to-out substrate-free to occluded state, to the open-to-in states (please see Supplementary Information file for full legend). (MOV 13215 kb)
Rights and permissions
About this article
Cite this article
Krishnamurthy, H., Gouaux, E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 481, 469–474 (2012). https://doi.org/10.1038/nature10737
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10737