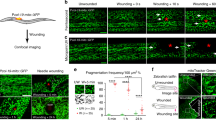
Abstract
Tissue wounding induces the rapid recruitment of leukocytes1. Wounds and tumours—a type of ‘unhealed wound’2—generate hydrogen peroxide (H2O2) through an NADPH oxidase (NOX). This extracellular H2O2 mediates recruitment of leukocytes, particularly the first responders of innate immunity, neutrophils, to injured tissue3,4,5,6. However, the sensor that neutrophils use to detect the redox state at wounds is unknown. Here we identify the Src family kinase (SFK) Lyn as a redox sensor that mediates initial neutrophil recruitment to wounds in zebrafish larvae. Lyn activation in neutrophils is dependent on wound-derived H2O2 after tissue injury, and inhibition of Lyn attenuates neutrophil wound recruitment. Inhibition of SFKs also disrupted H2O2-mediated chemotaxis of primary human neutrophils. In vitro analysis identified a single cysteine residue, C466, as being responsible for direct oxidation-mediated activation of Lyn. Furthermore, transgenic-tissue-specific reconstitution with wild-type Lyn and a cysteine mutant revealed that Lyn C466 is important for the neutrophil wound response and downstream signalling in vivo. This is the first identification, to our knowledge, of a physiological redox sensor that mediates leukocyte wound attraction in multicellular organisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nathan, C. Neutrophils and immunity: challenges and opportunities. Nature Rev. Immunol. 6, 173–182 (2006)
Dvorak, H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315, 1650–1659 (1986)
Klyubin, I. V., Kirpichnikova, K. M. & Gamaley, I. A. Hydrogen peroxide-induced chemotaxis of mouse peritoneal neutrophils. Eur. J. Cell Biol. 70, 347–351 (1996)
Feng, Y., Santoriello, C., Mione, M., Hurlstone, A. & Martin, P. Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: parallels between tumor initiation and wound inflammation. PLoS Biol. 8, e1000562 (2010)
Moreira, S., Stramer, B., Evans, I., Wood, W. & Martin, P. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr. Biol. 20, 464–470 (2010)
Niethammer, P., Grabher, C., Look, A. T. & Mitchison, T. J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459, 996–999 (2009)
Rhee, S. G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 312, 1882–1883 (2006)
Bienert, G. P., Schjoerring, J. K. & Jahn, T. P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 1758, 994–1003 (2006)
Paulsen, C. E. & Carroll, K. S. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem. Biol. 5, 47–62 (2010)
Poole, L. B. & Nelson, K. J. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr. Opin. Chem. Biol. 12, 18–24 (2008)
Miller, E. W., Dickinson, B. C. & Chang, C. J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl Acad. Sci. USA 107, 15681–15686 (2010)
Burgoyne, J. R. et al. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 317, 1393–1397 (2007)
Giannoni, E., Buricchi, F., Raugei, G., Ramponi, G. & Chiarugi, P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol. Cell. Biol. 25, 6391–6403 (2005)
Guo, Z., Kozlov, S., Lavin, M. F., Person, M. D. & Paull, T. T. ATM activation by oxidative stress. Science 330, 517–521 (2010)
Kemble, D. J. & Sun, G. Direct and specific inactivation of protein tyrosine kinases in the Src and FGFR families by reversible cysteine oxidation. Proc. Natl Acad. Sci. USA 106, 5070–5075 (2009)
Mathias, J. R. et al. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 80, 1281–1288 (2006)
Tobin, D. M. et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell 140, 717–730 (2010)
Yoo, S. K. et al. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev. Cell 18, 226–236 (2010)
Yoo, S. K. & Huttenlocher, A. Spatiotemporal photolabeling of neutrophil trafficking during inflammation in live zebrafish. J. Leukoc. Biol. 89, 661–667 (2011)
Martin, G. S. The hunting of the Src. Nature Rev. Mol. Cell Biol. 2, 467–475 (2001)
Sicheri, F., Moarefi, I. & Kuriyan, J. Crystal structure of the Src family tyrosine kinase Hck. Nature 385, 602–609 (1997)
Xu, W., Harrison, S. C. & Eck, M. J. Three-dimensional structure of the tyrosine kinase c-Src. Nature 385, 595–602 (1997)
Yeatman, T. J. A renaissance for SRC. Nature Rev. Cancer 4, 470–480 (2004)
Scapini, P., Pereira, S., Zhang, H. & Lowell, C. A. Multiple roles of Lyn kinase in myeloid cell signaling and function. Immunol. Rev. 228, 23–40 (2009)
Hibbs, M. L. et al. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell 83, 301–311 (1995)
Nishizumi, H. et al. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity 3, 549–560 (1995)
Pereira, S. & Lowell, C. The Lyn tyrosine kinase negatively regulates neutrophil integrin signaling. J. Immunol. 171, 1319–1327 (2003)
Lee, Y. M. et al. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell. Signal. 18, 499–507 (2006)
Abe, J., Takahashi, M., Ishida, M., Lee, J. D. & Berk, B. C. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J. Biol. Chem. 272, 20389–20394 (1997)
Yan, S. R. & Berton, G. Regulation of Src family tyrosine kinase activities in adherent human neutrophils. Evidence that reactive oxygen intermediates produced by adherent neutrophils increase the activity of the p58c-fgr and p53/56lyn tyrosine kinases. J. Biol. Chem. 271, 23464–23471 (1996)
Mathias, J. R. et al. Characterization of zebrafish larval inflammatory macrophages. Dev. Comp. Immunol. 33, 1212–1217 (2009)
Bennett, C. M. et al. Myelopoiesis in the zebrafish, Danio rerio. Blood 98, 643–651 (2001)
Thisse, C. & Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nature Protocols 3, 59–69 (2008)
Urasaki, A., Morvan, G. & Kawakami, K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics 174, 639–649 (2006)
Belousov, V. V. et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nature Methods 3, 281–286 (2006)
Chan, K. T., Cortesio, C. L. & Huttenlocher, A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J. Cell Biol. 185, 357–370 (2009)
Wong, B. R. et al. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol. Cell 4, 1041–1049 (1999)
Yamanashi, Y. et al. Activation of Src-like protein-tyrosine kinase Lyn and its association with phosphatidylinositol 3-kinase upon B-cell antigen receptor-mediated signaling. Proc. Natl Acad. Sci. USA 89, 1118–1122 (1992)
Heit, B. & Kubes, P. Measuring chemotaxis and chemokinesis: the under-agarose cell migration assay. Sci. STKE 2003, pl5 (2003)
Acknowledgements
We thank K. T. Chan for help with tissue culture work, J. M. Green and P.-Y. Lam for help with in situ hybridization, M. Shelef and S. Wernimont for drawing blood, and A. J. Wiemer for insightful discussion and critical reading of the manuscript. This work was supported by American Heart Association fellowship 11PRE4890041 (S.K.Y.), National Institutes of Health Grant GM074827 (A.H.), NIH Research Training Grant in Hematology 5T32 HL07899 (T.W.S.) and UW MSTP (T.W.S.).
Author information
Authors and Affiliations
Contributions
S.K.Y. designed the research, performed the experiments, analysed data and wrote the paper. T.W.S. contributed to development and data analysis of the in vitro chemotaxis assay. Q.D. constructed the HyPer probe and contributed expertise in zebrafish injection. A.H. designed the research, analysed data and co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-13 with legends, legends for Supplementary Movies 1-4 and additional references. (PDF 4881 kb)
Supplementary Movie 1
This movie shows time-lapse imaging of H2O2 immediately after tail transection in 2.5 dpf larvae expressing HyPer probe - see Supplementary Information file for full legend. (MOV 5055 kb)
Supplementary Movie 2
This movie shows time-lapse imaging of neutrophil random migration in the cephalic mesenchyme of 3 dpf Tg(mpx:Dendra2) - see Supplementary Information file for full legend. (MOV 3478 kb)
Supplementary Movie 3
This movie shows four examples of representative time-lapse imaging of 2.5 dpf Tg(mpx:Dendra2) injected with lyn MO or buffer - see Supplementary Information file for full legend. (MOV 712 kb)
Supplementary Movie 4
This movie shows LTB4-mediated neutrophil dissemination into the fins of zebrafish larvae - see Supplementary Information file for full legend. (MOV 2627 kb)
Rights and permissions
About this article
Cite this article
Yoo, S., Starnes, T., Deng, Q. et al. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 480, 109–112 (2011). https://doi.org/10.1038/nature10632
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10632
This article is cited by
-
Loss of Myo19 increases metastasis by enhancing microenvironmental ROS gradient and chemotaxis
EMBO Reports (2024)
-
A genetically encoded sensor for visualizing leukotriene B4 gradients in vivo
Nature Communications (2023)
-
Ontogenetically distinct neutrophils differ in function and transcriptional profile in zebrafish
Nature Communications (2023)
-
The engine initiating tissue regeneration: does a common mechanism exist during evolution?
Cell Regeneration (2021)
-
From wound response to repair – lessons from C. elegans
Cell Regeneration (2021)