Abstract
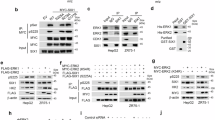
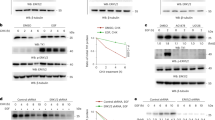
The embryonic pyruvate kinase M2 (PKM2) isoform is highly expressed in human cancer. In contrast to the established role of PKM2 in aerobic glycolysis or the Warburg effect1,2,3, its non-metabolic functions remain elusive. Here we demonstrate, in human cancer cells, that epidermal growth factor receptor (EGFR) activation induces translocation of PKM2, but not PKM1, into the nucleus, where K433 of PKM2 binds to c-Src-phosphorylated Y333 of β-catenin. This interaction is required for both proteins to be recruited to the CCND1 promoter, leading to HDAC3 removal from the promoter, histone H3 acetylation and cyclin D1 expression. PKM2-dependent β-catenin transactivation is instrumental in EGFR-promoted tumour cell proliferation and brain tumour development. In addition, positive correlations have been identified between c-Src activity, β-catenin Y333 phosphorylation and PKM2 nuclear accumulation in human glioblastoma specimens. Furthermore, levels of β-catenin phosphorylation and nuclear PKM2 have been correlated with grades of glioma malignancy and prognosis. These findings reveal that EGF induces β-catenin transactivation via a mechanism distinct from that induced by Wnt/Wingless4 and highlight the essential non-metabolic functions of PKM2 in EGFR-promoted β-catenin transactivation, cell proliferation and tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
01 December 2011
A minor text correction was made in paragraph beginning, 'PCR-amplified human PKM1 was cloned into...'.
References
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009)
Cairns, R. A., Harris, I. S. & Mak, T. W. Regulation of cancer cell metabolism. Nature Rev. Cancer 11, 85–95 (2011)
Koppenol, W. H., Bounds, P. L. & Dang, C. V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nature Rev. Cancer 11, 325–337 (2011)
Lu, Z. & Hunter, T. Wnt-independent β-catenin transactivation in tumor development. Cell Cycle 3, 569–571 (2004)
Lu, Z., Jiang, G., Blume-Jensen, P. & Hunter, T. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol. Cell. Biol. 21, 4016–4031 (2001)
Wykosky, J., Fenton, T., Furnari, F. & Cavenee, W. K. Therapeutic targeting of epidermal growth factor receptor in human cancer: successes and limitations. Chin. J. Cancer 30, 5–12 (2011)
Christofk, H. R. et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452, 230–233 (2008)
Yochum, G. S. et al. Serial analysis of chromatin occupancy identifies β-catenin target genes in colorectal carcinoma cells. Proc. Natl Acad. Sci. USA 104, 3324–3329 (2007)
Christofk, H. R., Vander Heiden, M. G., Wu, N., Asara, J. M. & Cantley, L. C. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 452, 181–186 (2008)
Le Mellay, V. et al. Regulation of glycolysis by Raf protein serine/threonine kinases. Adv. Enzyme Regul. 42, 317–332 (2002)
Mazurek, S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int. J. Biochem. Cell Biol. 43, 969–980 (2011)
Coluccia, A. M. et al. Bcr-Abl stabilizes β-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 26, 1456–1466 (2007)
Miravet, S. et al. Tyrosine phosphorylation of plakoglobin causes contrary effects on its association with desmosomes and adherens junction components and modulates β-catenin-mediated transcription. Mol. Cell. Biol. 23, 7391–7402 (2003)
Xia, Y. et al. c-Jun downregulation by HDAC3-dependent transcriptional repression promotes osmotic stress-induced cell apoptosis. Mol. Cell 25, 219–232 (2007)
Tetsu, O. & McCormick, F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398, 422–426 (1999)
Freije, W. A. et al. Gene expression profiling of gliomas strongly predicts survival. Cancer Res. 64, 6503–6510 (2004)
Gravendeel, L. A. et al. Intrinsic gene expression profiles of gliomas are a better predictor of survival than histology. Cancer Res. 69, 9065–9072 (2009)
Petalidis, L. P. et al. Improved grading and survival prediction of human astrocytic brain tumors by artificial neural network analysis of gene expression microarray data. Mol. Cancer Ther. 7, 1013–1024 (2008)
Phillips, H. S. et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9, 157–173 (2006)
Furnari, F. B. et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 21, 2683–2710 (2007)
Ji, H. et al. EGF-induced ERK activation promotes CK2-mediated disassociation of α-catenin from β-catenin and transactivation of β-catenin. Mol. Cell 36, 547–559 (2009)
Fang, D. et al. Phosphorylation of β-catenin by AKT promotes β-catenin transcriptional activity. J. Biol. Chem. 282, 11221–11229 (2007)
Lu, Z., Ghosh, S., Wang, Z. & Hunter, T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion. Cancer Cell 4, 499–515 (2003)
Luo, W. et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145, 732–744 (2011)
Lu, Z. et al. Activation of protein kinase C triggers its ubiquitination and degradation. Mol. Cell. Biol. 18, 839–845 (1998)
Gomez-Manzano, C. et al. Delta-24 increases the expression and activity of topoisomerase I and enhances the antiglioma effect of irinotecan. Clin. Cancer Res. 12, 556–562 (2006)
Acknowledgements
We thank T. Hunter (The Salk Institute for Biological Studies) for Abl and Src knockout cells, H. Clevers (Netherlands Institute for Developmental Biology) for the pTOP-FLASH and the pFOP-FLASH, and Y. Li (Baylor College of Medicine) for a WNT1 lenti-vector. This work was supported by National Cancer Institute grants 5R01CA109035 (Z.L.), 5 P50 CA127001-03 and CA16672 (Cancer Center Support Grant); a research grant (RP110252; Z.L.) from the Cancer Prevention and Research Institute of Texas (CPRIT), an American Cancer Society Research Scholar Award RSG-09-277-01-CSM (Z.L.), and a Sister Institution Network Fund from The University of Texas MD Anderson Cancer Center (Z.L.).
Author information
Authors and Affiliations
Contributions
This study was conceived by Z.L. Z.L and W.Y. designed the study; W.Y., Y.X., H.J., Y.Z. and J.L. performed experiments; K.A. provided pathology assistance; W.H. and X.G. provided reagents and conceptual advice; Z.L. wrote the paper with comments from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-21 with legends, a Supplementary Discussion and Supplementary References. (PDF 8004 kb)
Rights and permissions
About this article
Cite this article
Yang, W., Xia, Y., Ji, H. et al. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature 480, 118–122 (2011). https://doi.org/10.1038/nature10598
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10598
This article is cited by
-
Fructose-1,6-bisphosphatase 1 dephosphorylates and inhibits TERT for tumor suppression
Nature Chemical Biology (2024)
-
MYG1 drives glycolysis and colorectal cancer development through nuclear-mitochondrial collaboration
Nature Communications (2024)
-
Epigenetic-based combination therapy and liposomal codelivery overcomes osimertinib-resistant NSCLC via repolarizing tumor-associated macrophages
Acta Pharmacologica Sinica (2024)
-
Silencing PKM2 Attenuates Brain Injury Induced by Status Epilepticus by Inhibiting the AKT/mTOR Pathway and the NLRP3 Inflammasome
Neurochemical Research (2024)
-
Metformin Increases the Response of Cholangiocarcinoma Cells to Gemcitabine by Suppressing Pyruvate Kinase M2 to Activate Mitochondrial Apoptosis
Digestive Diseases and Sciences (2024)