Abstract
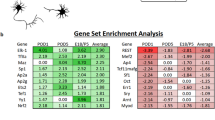
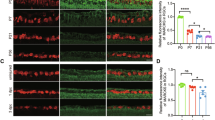
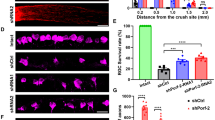
A formidable challenge in neural repair in the adult central nervous system (CNS) is the long distances that regenerating axons often need to travel in order to reconnect with their targets. Thus, a sustained capacity for axon regeneration is critical for achieving functional restoration. Although deletion of either phosphatase and tensin homologue (PTEN), a negative regulator of mammalian target of rapamycin (mTOR), or suppressor of cytokine signalling 3 (SOCS3), a negative regulator of Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway, in adult retinal ganglion cells (RGCs) individually promoted significant optic nerve regeneration, such regrowth tapered off around 2 weeks after the crush injury1,2. Here we show that, remarkably, simultaneous deletion of both PTEN and SOCS3 enables robust and sustained axon regeneration. We further show that PTEN and SOCS3 regulate two independent pathways that act synergistically to promote enhanced axon regeneration. Gene expression analyses suggest that double deletion not only results in the induction of many growth-related genes, but also allows RGCs to maintain the expression of a repertoire of genes at the physiological level after injury. Our results reveal concurrent activation of mTOR and STAT3 pathways as key for sustaining long-distance axon regeneration in adult CNS, a crucial step towards functional recovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Data deposits
Microarray data are deposited in Gene Expression Omnibus under accession number GSE32309.
References
Park, K. K. et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322, 963–966 (2008)
Smith, P. D. et al. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron 64, 617–623 (2009)
Fawcett, J. Molecular control of brain plasticity and repair. Prog. Brain Res. 175, 501–509 (2009)
Filbin, M. T. Recapitulate development to promote axonal regeneration: good or bad approach? Phil. Trans. R. Soc. B 361, 1565–1574 (2006)
Fitch, M. T. & Silver, J. CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 209, 294–301 (2008)
Hellal, F. et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science 331, 928–931 (2011)
Leibinger, M. et al. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J. Neurosci. 29, 14334–14341 (2009)
Moore, D. L. et al. KLF family members regulate intrinsic axon regeneration ability. Science 326, 298–301 (2009)
Winzeler, A. M. et al. The lipid sulfatide is a novel myelin-associated inhibitor of CNS axon outgrowth. J. Neurosci. 31, 6481–6492 (2011)
Groszer, M. et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science 294, 2186–2189 (2001)
Mori, H. et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nature Med. 10, 739–743 (2004)
Fasnacht, N. & Muller, W. Conditional gp130 deficient mouse mutants. Semin. Cell Dev. Biol. 19, 379–384 (2008)
Ernst, M. & Jenkins, B. J. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 20, 23–32 (2004)
Park, K. K. et al. Cytokine-induced SOCS expression is inhibited by cAMP analogue: impact on regeneration in injured retina. Mol. Cell. Neurosci. 41, 313–324 (2009)
Park, K., Luo, J. M., Hisheh, S., Harvey, A. R. & Cui, Q. Cellular mechanisms associated with spontaneous and ciliary neurotrophic factor-cAMP-induced survival and axonal regeneration of adult retinal ganglion cells. J. Neurosci. 24, 10806–10815 (2004)
Bareyre, F. M. et al. In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc. Natl Acad. Sci. USA 108, 6282–6287 (2011)
Miao, T. et al. Suppressor of cytokine signaling-3 suppresses the ability of activated signal transducer and activator of transcription-3 to stimulate neurite growth in rat primary sensory neurons. J. Neurosci. 26, 9512–9519 (2006)
Qiu, J., Cafferty, W. B., McMahon, S. B. & Thompson, S. W. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J. Neurosci. 25, 1645–1653 (2005)
Aaronson, D. S. & Horvath, C. M. A road map for those who don’t know JAK-STAT. Science 296, 1653–1655 (2002)
Sengupta, S., Peterson, T. R. & Sabatini, D. M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 40, 310–322 (2010)
Joset, P. et al. Rostral growth of commissural axons requires the cell adhesion molecule MDGA2. Neural Develop. 6, 22 (2011)
Junghans, D., Haas, I. G. & Kemler, R. Mammalian cadherins and protocadherins: about cell death, synapses and processing. Curr. Opin. Cell Biol. 17, 446–452 (2005)
Low, K., Culbertson, M., Bradke, F., Tessier-Lavigne, M. & Tuszynski, M. H. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J. Neurosci. 28, 1099–1108 (2008)
Hannila, S. S. & Filbin, M. T. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp. Neurol. 209, 321–332 (2008)
Nix, P., Hisamoto, N., Matsumoto, K. & Bastiani, M. Axon regeneration requires coordinate activation of p38 and JNK MAPK pathways. Proc. Natl Acad. Sci. USA 108, 10738–10743 (2011)
Hanz, S. & Fainzilber, M. Retrograde signaling in injured nerve–the axon reaction revisited. J. Neurochem. 99, 13–19 (2006)
Hoffman, P. N. A conditioning lesion induces changes in gene expression and axonal transport that enhance regeneration by increasing the intrinsic growth state of axons. Exp. Neurol. 223, 11–18 (2010)
Park, K. K., Liu, K., Hu, Y., Kanter, J. L. & He, Z. PTEN/mTOR and axon regeneration. Exp. Neurol. 223, 45–50 (2010)
Abe, N., Borson, S. H., Gambello, M. J., Wang, F. & Cavalli, V. Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. J. Biol. Chem. 285, 28034–28043 (2010)
Christie, K. J., Webber, C. A., Martinez, J. A., Singh, B. & Zochodne, D. W. PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J. Neurosci. 30, 9306–9315 (2010)
Acknowledgements
We thank M. Curry and C. Wang for technical support, H. Sasaki and F. Wang for providing Stat3f/f and Rosa-lox-STOP-lox-Tomato mice, J. Gray, M. Hemberg, J. Choi, J. Ngai and W. Wang for advice on microarray and data analysis, and J. Gray, X. He, T. Schwarz, F. Wang, W. Wang and C. Woolf for reading the manuscript. This study was supported by grants from Wings for Life (to F.S.), Miami Project to Cure Paralysis (to K.K.P.) and NEI (to Z.H.).
Author information
Authors and Affiliations
Contributions
F.S, K.K.P., K.Z. and Z.H. conceived and F.S., K.K.P., S.B., G.C. and C.Y. performed the experiments. D.W. and G.F. provided YFP-17 mice, F.S., T.L., B.A.Y. and Z.H. analysed gene array data. F.S., K.K.P. and Z.H. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
Z.H. is a co-founder of Axonis.
Supplementary information
Supplementary Figures
The file contains Supplementary Figures 1-12 with legends. (PDF 2817 kb)
Rights and permissions
About this article
Cite this article
Sun, F., Park, K., Belin, S. et al. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 480, 372–375 (2011). https://doi.org/10.1038/nature10594
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10594