Abstract
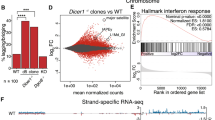
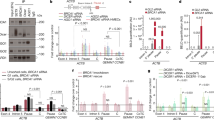
Mutations in the tumour suppressor gene BRCA1 lead to breast and/or ovarian cancer. Here we show that loss of Brca1 in mice results in transcriptional de-repression of the tandemly repeated satellite DNA. Brca1 deficiency is accompanied by a reduction of condensed DNA regions in the genome and loss of ubiquitylation of histone H2A at satellite repeats. BRCA1 binds to satellite DNA regions and ubiquitylates H2A in vivo. Ectopic expression of H2A fused to ubiquitin reverses the effects of BRCA1 loss, indicating that BRCA1 maintains heterochromatin structure via ubiquitylation of histone H2A. Satellite DNA de-repression was also observed in mouse and human BRCA1-deficient breast cancers. Ectopic expression of satellite DNA can phenocopy BRCA1 loss in centrosome amplification, cell-cycle checkpoint defects, DNA damage and genomic instability. We propose that the role of BRCA1 in maintaining global heterochromatin integrity accounts for many of its tumour suppressor functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tutt, A. & Ashworth, A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol. Med. 8, 571–576 (2002)
King, M. C., Marks, J. H. & Mandell, J. B. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 302, 643–646 (2003)
Huen, M. S., Sy, S. M. & Chen, J. BRCA1 and its toolbox for the maintenance of genome integrity. Nature Rev. Mol. Cell Biol. 11, 138–148 (2010)
Pageau, G. J., Hall, L. L., Ganesan, S., Livingston, D. M. & Lawrence, J. B. The disappearing Barr body in breast and ovarian cancers. Nature Rev. Cancer 7, 628–633 (2007)
Lane, T. F. et al. Expression of Brca1 is associated with terminal differentiation of ectodermally and mesodermally derived tissues in mice. Genes Dev. 9, 2712–2722 (1995)
Korhonen, L., Brannvall, K., Skoglosa, Y. & Lindholm, D. Tumor suppressor gene BRCA-1 is expressed by embryonic and adult neural stem cells and involved in cell proliferation. J. Neurosci. Res. 71, 769–776 (2003)
Maison, C. & Almouzni, G. HP1 and the dynamics of heterochromatin maintenance. Nature Rev. Mol. Cell Biol. 5, 296–305 (2004)
Xia, Y., Pao, G. M., Chen, H. W., Verma, I. M. & Hunter, T. Enhancement of BRCA1 E3 ubiquitin ligase activity through direct interaction with the BARD1 protein. J. Biol. Chem. 278, 5255–5263 (2003)
Wang, H. et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature 431, 873–878 (2004)
Meneghini, M. D., Wu, M. & Madhani, H. D. Conserved histone variant H2A.Z protects euchromatin from the ectopic spread of silent heterochromatin. Cell 112, 725–736 (2003)
Martens, J. H. et al. The profile of repeat-associated histone lysine methylation states in the mouse epigenome. EMBO J. 24, 800–812 (2005)
Hashizume, R. et al. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J. Biol. Chem. 276, 14537–14540 (2001)
Reid, L. J. et al. E3 ligase activity of BRCA1 is not essential for mammalian cell viability or homology-directed repair of double-strand DNA breaks. Proc. Natl Acad. Sci. USA 105, 20876–20881 (2008)
Xu, X. et al. Genetic interactions between tumor suppressors Brca1 and p53 in apoptosis, cell cycle and tumorigenesis. Nature Genet. 28, 266–271 (2001)
Sankaran, S., Starita, L. M., Groen, A. C., Ko, M. J. & Parvin, J. D. Centrosomal microtubule nucleation activity is inhibited by BRCA1-dependent ubiquitination. Mol. Cell. Biol. 25, 8656–8668 (2005)
Matsui, S. I., Seon, B. K. & Sandberg, A. A. Disappearance of a structural chromatin protein A24 in mitosis: implications for molecular basis of chromatin condensation. Proc. Natl Acad. Sci. USA 76, 6386–6390 (1979)
Kallin, E. M. et al. Genome-wide uH2A localization analysis highlights Bmi1-dependent deposition of the mark at repressed genes. PLoS Genet. 5, e1000506 (2009)
Zhou, W. et al. Histone H2A monoubiquitination represses transcription by inhibiting RNA polymerase II transcriptional elongation. Mol. Cell 29, 69–80 (2008)
Doil, C. et al. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell 136, 435–446 (2009)
Stewart, G. S. et al. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell 136, 420–434 (2009)
Scheuermann, J. C. et al. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature 465, 243–247 (2010)
Jensen, D. E. et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 16, 1097–1112 (1998)
Ruffner, H., Joazeiro, C. A. P., Hemmati, D., Hunter, T. & Verma, I. M. Cancer-predisposing mutations within the RING domain of BRCA1: Loss of ubiquitin protein ligase activity and protection from radiation hypersensitivity. Proc. Natl Acad. Sci. USA 98, 5134–5139 (2001)
Ting, D. T. et al. Aberrant overexpression of satellite repeats in pancreatic and other epithelial cancers. Science 331, 593–596 (2011)
Palmer, T. D., Markakis, E. A., Willhoite, A. R., Safar, F. & Gage, F. H. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J. Neurosci. 19, 8487–8497 (1999)
Nakashima, K. et al. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 284, 479–482 (1999)
van Es, J. H. et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nature Cell Biol. 7, 381–386 (2005)
Lie, D. C. et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 437, 1370–1375 (2005)
Huang, J. et al. Lsh, an epigenetic guardian of repetitive elements. Nucleic Acids Res. 32, 5019–5028 (2004)
Acknowledgements
We thank B. Miller for assistance in culturing NPCs; E. Ke for discussion and analysis of Affymetrix data; A. Yanai for the BRCA1 targeting shRNA construct; C. Lilley for assistance with western blotting; and Z. You for assistance with the LI-COR Odyssey Infrared Imaging System. We thank A. Berns for his sustained interest in this work and providing mutant mice and materials, and M. Vidal for providing mouse embryo fibroblasts containing a conditional deletion allele of Ring1B. Q.Z. was supported by the California Breast Cancer Research Program and Ruth L. Kirschtein National Research Service Award. G.M.P. was supported by a fellowship of the California Institute of Regenerative Medicine. H.S. is a recipient of ASPET-Merck fellowship. I.M.V. is an American Cancer Society Professor of Molecular Biology, and holds the Irwin and Joan Jacobs Chair in Exemplary Life Science. This work was supported in part by grants from the NIH, Ipsen/Biomeasure, Sanofi Aventis, and the H.N. and Frances C. Berger Foundation. F.H.G. is supported by NIH NS52842, NS50217 and the Lookout Fund.
Author information
Authors and Affiliations
Contributions
Q.Z. generated and Q.Z., N.T. and G.M.P. maintained all the knockout mice. G.M.P. and Q.Z. made the initial heterochromatin observation. Q.Z. and A.M.H. performed confocal microscopy experiments. G.M.P., Q.Z. and N.T. performed ChIP experiments. RNA isolation and microarray experiments were performed by Q.Z., G.M.P. and N.T. Microdissection of murine brains were performed by G.M.P. and A.M.H. under the guidance of F.H.G. G.M.P. designed the H2A–ubiquitin fusion experiments that were performed by Q.Z., G.M.P. and N.T. Satellite RNA experiments were designed by G.M.P. and Q.Z. and performed by Q.Z., G.M.P. and N.T. H.S. established the embryonic neural stem cell isolation and culture. P.M.N. obtained, isolated and curated the clinical patient samples, which were analysed by Q.Z., G.M.P. and N.T. All other experiments were performed by Q.Z., G.M.P. and N.T. All experiments and experimental design was performed under the supervision of I.M.V. G.M.P., Q.Z. and I.M.V. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
The file contains Supplementary Figures 1-14 with legends. (PDF 1249 kb)
Rights and permissions
About this article
Cite this article
Zhu, Q., Pao, G., Huynh, A. et al. BRCA1 tumour suppression occurs via heterochromatin-mediated silencing. Nature 477, 179–184 (2011). https://doi.org/10.1038/nature10371
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10371
This article is cited by
-
Inheritance of H3K9 methylation regulates genome architecture in Drosophila early embryos
The EMBO Journal (2024)
-
Redox-dependent Igfbp2 signaling controls Brca1 DNA damage response to govern neural stem cell fate
Nature Communications (2023)
-
TRIM22 promotes the proliferation of glioblastoma cells by activating MAPK signaling and accelerating the degradation of Raf-1
Experimental & Molecular Medicine (2023)
-
Histone H3K9 methyltransferase SETDB1 overexpression correlates with pediatric high-grade gliomas progression and prognosis
Journal of Molecular Medicine (2023)
-
Overexpression of satellite RNAs in heterochromatin induces chromosomal instability and reflects drug sensitivity in mouse cancer cells
Scientific Reports (2022)