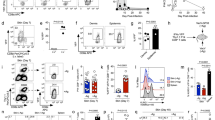
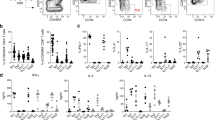
Abstract
Infections localized to peripheral tissues such as the skin result in the priming of T-cell responses that act to control pathogens. Activated T cells undergo migrational imprinting within the draining lymph nodes1, resulting in memory T cells that provide local and systemic protection2. Combinations of migrating and resident memory T cells have been implicated in long-term peripheral immunity, especially at the surfaces that form pathogen entry points into the body3. However, T-cell immunity consists of separate CD4+ helper T cells and CD8+ killer T cells, with distinct effector and memory programming requirements4. Whether these subsets also differ in their ability to form a migrating pool involved in peripheral immunosurveillance or a separate resident population responsible for local infection control has not been explored. Here, using mice, we show key differences in the migration and tissue localization of memory CD4+ and CD8+ T cells following infection of the skin by herpes simplex virus. On resolution of infection, the skin contained two distinct virus-specific memory subsets; a slow-moving population of sequestered CD8+ T cells that were resident in the epidermis and confined largely to the original site of infection, and a dynamic population of CD4+ T cells that trafficked rapidly through the dermis as part of a wider recirculation pattern. Unique homing-molecule expression by recirculating CD4+ T effector-memory cells mirrored their preferential skin-migratory capacity. Overall, these results identify a complexity in memory T-cell migration, illuminating previously unappreciated differences between the CD4+ and CD8+ subsets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sigmundsdottir, H. & Butcher, E. C. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nature Immunol. 9, 981–987 (2008)
Kaech, S. M. & Wherry, E. J. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 27, 393–405 (2007)
Woodland, D. L. & Kohlmeier, J. E. Migration, maintenance and recall of memory T cells in peripheral tissues. Nature Rev. Immunol. 9, 153–161 (2009)
Seder, R. A. & Ahmed, R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nature Immunol. 4, 835–842 (2003)
Bousso, P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nature Rev. Immunol. 8, 675–684 (2008)
Mueller, S. N. & Hickman, H. D. In vivo imaging of the T cell response to infection. Curr. Opin. Immunol. 22, 293–298 (2010)
Bedoui, S. et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nature Immunol. 10, 488–495 (2009)
Mueller, S. N., Heath, W., McLain, J. D., Carbone, F. R. & Jones, C. M. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol. Cell Biol. 80, 156–163 (2002)
van Lint, A. et al. Herpes simplex virus-specific CD8+ T cells can clear established lytic infections from skin and nerves and can partially limit the early spread of virus after cutaneous inoculation. J. Immunol. 172, 392–397 (2004)
Cepek, K. L. et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature 372, 190–193 (1994)
Schon, M. P. et al. Mucosal T lymphocyte numbers are selectively reduced in integrin αE (CD103)-deficient mice. J. Immunol. 162, 6641–6649 (1999)
Nestle, F. O., Di Meglio, P., Qin, J. Z. & Nickoloff, B. J. Skin immune sentinels in health and disease. Nature Rev. Immunol. 9, 679–691 (2009)
Zhu, J. et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nature Med. 15, 886–892 (2009)
Miller, C. J., McChesney, M. & Moore, P. F. Langerhans cells, macrophages and lymphocyte subsets in the cervix and vagina of rhesus macaques. Lab. Invest. 67, 628–634 (1992)
Mackay, C. R., Marston, W. L. & Dudler, L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J. Exp. Med. 171, 801–817 (1990)
Yawalkar, N., Hunger, R. E., Pichler, W. J., Braathen, L. R. & Brand, C. U. Human afferent lymph from normal skin contains an increased number of mainly memory/effector CD4+ T cells expressing activation, adhesion and co-stimulatory molecules. Eur. J. Immunol. 30, 491–497 (2000)
Ley, K., Laudanna, C., Cybulsky, M. I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Rev. Immunol. 7, 678–689 (2007)
Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001)
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999)
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature Immunol. 10, 524–530 (2009)
Wakim, L. M., Jones, C. M., Gebhardt, T., Preston, C. M. & Carbone, F. R. CD8+ T-cell attenuation of cutaneous herpes simplex virus infection reduces the average viral copy number of the ensuing latent infection. Immunol. Cell Biol. 86, 666–675 (2008)
Masopust, D. et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207, 553–564 (2010)
Wakim, L. M., Woodward-Davis, A. & Bevan, M. J. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl Acad. Sci. USA 107, 17872–17879 (2010)
Klonowski, K. D. et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 20, 551–562 (2004)
Stock, A. T., Jones, C. M., Heath, W. R. & Carbone, F. R. Rapid recruitment and activation of CD8+ T cells after herpes simplex virus type 1 skin infection. Immunol. Cell Biol. 89, 143–148 (2011)
Acknowledgements
We thank members of the Carbone and Heath laboratories for discussions. This work was supported by the Australian NHMRC and ARC.
Author information
Authors and Affiliations
Contributions
T.G., W.R.H., F.R.C. and S.N.M. designed the study. T.G., A.Z., L.K.M. and S.N.M. performed experiments. T.G., A.Z. and S.N.M. collected and analysed data. P.G.W. and A.G.B. provided reagents and gave conceptual advice. F.R.C. wrote the manuscript with T.G., W.R.H. and S.N.M. The research program was led by F.R.C. and W.R.H. who are joint senior authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-10 with legends, and Supplementary Movie legends 1-6. (PDF 4454 kb)
Supplementary Movie 1
This movie shows the migration of effector CD8+ T cells and CD4+ T cells in the epidermis and dermis of the skin 8 days after HSV skin infection of mice (see Supplementary Information file for full legend). (MOV 4182 kb)
Supplementary Movie 2
This movie demonstrates the migratory tracks of effector CD8+ T cells and CD4+ T cells in the epidermis and dermis of the skin 8 days after HSV skin infection (see Supplementary Information file for full legend). (MOV 5974 kb)
Supplementary Movie 3
This movie shows the differential localisation of memory CD8+ T cells in the epidermis and memory CD4+ T cells in the dermis of the skin after HSV skin infection. The dendritic morphology and low speed migration of the epidermal CD8+ T cells is evident alongside rapidly migrating dermal CD4+ T cells (see Supplementary Information file for full legend). (MOV 2409 kb)
Supplementary Movie 4
This movie further demonstrates the differential localization and migration by memory CD8+ T cells in the epidermis and memory CD4+ T cells in the dermis of the skin after HSV skin infection (see Supplementary Information file for full legend). (MOV 3284 kb)
Supplementary Movie 5
This movie shows the tracking of memory CD8+ and CD4+ T cells in the skin after HSV skin infection, and further highlights the dendritic morphology and low speed migration of the epidermal CD8+ T cells (see Supplementary Information file for full legend). (MOV 5329 kb)
Supplementary Movie 6
This movie demonstrates that memory CD4+ T cells continue to migrate dynamically through skin, at sites distal to the infection (see Supplementary Information file for full legend). (MOV 2254 kb)
Rights and permissions
About this article
Cite this article
Gebhardt, T., Whitney, P., Zaid, A. et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477, 216–219 (2011). https://doi.org/10.1038/nature10339
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10339
This article is cited by
-
CD4+ T cell memory
Nature Immunology (2023)
-
Assessing the generation of tissue resident memory T cells by vaccines
Nature Reviews Immunology (2023)
-
Unique properties of tissue-resident memory T cells in the lungs: implications for COVID-19 and other respiratory diseases
Nature Reviews Immunology (2023)
-
Langerhans cells shape postnatal oral homeostasis in a mechanical-force-dependent but microbiota and IL17-independent manner
Nature Communications (2023)
-
IKK2/NFkB signaling controls lung resident CD8+ T cell memory during influenza infection
Nature Communications (2023)