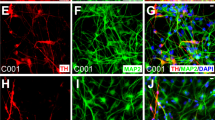
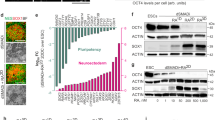
Abstract
Transplantation of dopaminergic neurons can potentially improve the clinical outcome of Parkinson’s disease, a neurological disorder resulting from degeneration of mesencephalic dopaminergic neurons1,2. In particular, transplantation of embryonic-stem-cell-derived dopaminergic neurons has been shown to be efficient in restoring motor symptoms in conditions of dopamine deficiency3,4. However, the use of pluripotent-derived cells might lead to the development of tumours if not properly controlled5. Here we identified a minimal set of three transcription factors—Mash1 (also known as Ascl1), Nurr1 (also known as Nr4a2) and Lmx1a—that are able to generate directly functional dopaminergic neurons from mouse and human fibroblasts without reverting to a progenitor cell stage. Induced dopaminergic (iDA) cells release dopamine and show spontaneous electrical activity organized in regular spikes consistent with the pacemaker activity featured by brain dopaminergic neurons. The three factors were able to elicit dopaminergic neuronal conversion in prenatal and adult fibroblasts from healthy donors and Parkinson’s disease patients. Direct generation of iDA cells from somatic cells might have significant implications for understanding critical processes for neuronal development, in vitro disease modelling and cell replacement therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
Gene Expression Omnibus
Data deposits
Data have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO series accession number GSE27174 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc5GSE27174).
References
Lindvall, O. & Björklund, A. Cell therapy in Parkinson’s disease. NeuroRx 1, 382–393 (2004)
Politis, M. et al. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci. Transl. Med. 2, 38–46 (2010)
Kim, J. H. et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature 418, 50–56 (2002)
Barberi, T. et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nature Biotechnol. 21, 1200–1207 (2003)
Wernig, M. et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc. Natl Acad. Sci. USA 105, 5856–5861 (2008)
Heins, N. et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nature Neurosci. 5, 308–315 (2002)
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010)
Ang, S.-L. Transcriptional control of midbrain dopaminergic neuron development. Development 133, 3499–3506 (2006)
Smidt, M. P. & Burbach, J. P. How to make a mesodiencephalic dopaminergic neuron. Nature Rev. Neurosci. 8, 21–32 (2007)
Sawamoto, K. et al. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc. Natl Acad. Sci. USA 98, 6423–6428 (2001)
Perlmann, T. & Wallén-Mackenzie, A. Nurr1, an orphan nuclear receptor with essential functions in developing dopamine cells. Cell Tissue Res. 318, 45–52 (2004)
Stadtfeld, M., Maherali, N., Breault, D. T. & Hochedlinger, K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell 2, 230–240 (2008)
Grace, A. A. & Bunney, B. S. The control of firing pattern in nigral dopamine neurons: single spike firing. J. Neurosci. 4, 2866–2876 (1984)
Grace, A. A. & Onn, S. P. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro . J. Neurosci. 9, 3463–3481 (1989)
Pothos, E. N., Davila, V. & Sulzer, D. Presynaptic recording of quanta from midbrain dopamine neurons and modulation of the quantal size. J. Neurosci. 18, 4106–4118 (1998)
Staal, R. G. W., Mosharov, E. V. & Sulzer, D. Dopamine neurons release transmitter via a flickering fusion pore. Nature Neurosci. 7, 341–346 (2004)
Simeone, A. Genetic control of dopaminergic neuron differentiation. Trends Neurosci. 28, 62–65 (2005)
Zappone, M. V. et al. Sox2 regulatory sequences direct expression of a β-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development 127, 2367–2382 (2000)
Pang, Z. P. et al. Induction of human neuronal cells by defined transcription factors. Nature advance online publication,. 10.1038/nature10202 (26 May 2011)
Pfisterer, U. et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl Acad. Sci. USA 10.1073/pnas.1105135108 (6 June 2011)
Amariglio, N. et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 6, e1000029 (2009)
Sironi, F. et al. α-Synuclein multiplication analysis in Italian familial Parkinson disease. Parkinsonism Relat. Disord. 16, 228–231 (2010)
Sironi, F. et al. Parkin analysis in early onset Parkinson’s disease. Parkinsonism Relat. Disord. 14, 326–333 (2008)
Pruszak, J. et al. Isolation and culture of ventral mesencephalic precursor cells and dopaminergic neurons from rodent brain. Curr. Prot. Stem Cell Biol. Chapter 2,. 2D.5.1–2D.5.21 (2009)
Broccoli, V. et al. The caudal limit of Otx2 expression positions the isthmic organizer. Nature 401, 164–168 (1999)
Irizarry, R. A. et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31, e15 (2003)
Smyth, G. K. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, Article 3. (2004)
Hochberg, Y. & Benjamini, Y. More powerful procedures for multiple significance testing. Stat. Med. 9, 811–818 (1990)
Huang, D. W. et al. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 (2009)
Biagioli, M. et al. Unexpected expression of α- and β-globin in mesencephalic dopaminergic neurons and glial cells. Proc. Natl Acad. USA 106, 15454–15459 (2009)
Pothos, E., Desmond, D. & Sulzer, D. l-3,4-Dihydroxyphenylalanine increases the quantal size of exocytotic dopamine release in vitro. J. Neurochem. 66, 629–636 (1996)
Mundroff, M. L. & Wightman, R. M. Amperometry and cyclic voltammetry with carbon fiber microelectrodes at single cells. Curr. Protoc. Neurosci. Chapter 6,. 6.14.1–6.14.22 (2002)
Menegon, A. et al. Protein kinase A-mediated synapsin I phosphorylation is a central modulator of Ca2+-dependent synaptic activity. J. Neurosci. 26, 11670–11681 (2006)
Colasante, G. et al. Arx is a direct target of Dlx2 and thereby contributes to the tangential migration of GABAergic interneurons. J. Neurosci. 28, 10674–10686 (2008)
West, M. J. et al. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 231, 482–497 (1991)
Bacigaluppi, M. et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain 132, 2239–2251 (2009)
Acknowledgements
We are thankful to D. Bonanomi, S.-L. Ang, S. El Mestikawy, M. P. Smidt, M. German and F. Valtorta for providing valuable antibodies. We thank A. Sessa and V.B. laboratory members for helpful discussion. M. Wernig is acknowledged for providing the iN-inducing lentiviral vectors. We are thankful to S. Nicolis for sharing Sox2β-geo mice. L. Muzio, C. Laterza and G. Martino are acknowledged for the generation of Sox2β-geo induced pluripotent stem cells. M. Bacigaluppi is acknowledged for advice on stereological countings. We thank the “Cell Line and DNA Biobank” (G. Gaslini Institute) and “Human Genetic Bank of Patients affected by Parkinson Disease and parkinsonism” (Parkinson Institute of Milan) of the Telethon Genetic Biobank Network for human fibroblast samples. This study was supported by the “Fondazione Grigioni per il Morbo di Parkinson” (grant no. FGBRCVNI10310-001-V.B.), Eranet Neuron (V.B.), Cariplo Foundation (V.B.), Ministry of Health (Giovani ricercatori Award) (V.B.) and Italian Institute of Technology (V.B., A.D., S.G., T.S., R.G.).
Author information
Authors and Affiliations
Contributions
M.C. and V.B. designed and conceived the experiments. M.C., M.T.D. and G.C. performed the lentiviral infections, characterized reprogrammed cells and analysed their fate after in vivo transplantation. E.D. and A.D. designed, performed and analysed all electrophysiological experiments. P.R., D.L., P.C. and S.G. performed the microarray gene expression profiling and analysed the data. D.L., A.D. and R.R.G. designed andD.L. andE.D. performed theamperometric experiments. R.R.G. and T.D.S. designed the protocol and performed the assessment of dopamine levels. S.T and G.R. performed patch-clamp recording on brain slices. A.M. performed the functional analysis of synaptic activity. G.P. supervised the selection of the Parkinson's disease patients and the isolation of the primary fibroblasts. V.B. and A.D. should be considered as co-senior authors and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
A patent application including the results of this manuscript has been filed, but has not been published yet.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-13 with legends, and legends for Supplementary Tables 1-6. (PDF 10440 kb)
Supplementary Tables
This file contains Supplementary Tables 1-6 (see Supplementary Information file for legends). (ZIP 321 kb)
Rights and permissions
About this article
Cite this article
Caiazzo, M., Dell’Anno, M., Dvoretskova, E. et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476, 224–227 (2011). https://doi.org/10.1038/nature10284
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10284