Abstract
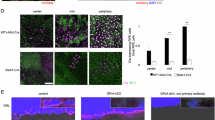
Geographic atrophy (GA), an untreatable advanced form of age-related macular degeneration, results from retinal pigmented epithelium (RPE) cell degeneration. Here we show that the microRNA (miRNA)-processing enzyme DICER1 is reduced in the RPE of humans with GA, and that conditional ablation of Dicer1, but not seven other miRNA-processing enzymes, induces RPE degeneration in mice. DICER1 knockdown induces accumulation of Alu RNA in human RPE cells and Alu-like B1 and B2 RNAs in mouse RPE. Alu RNA is increased in the RPE of humans with GA, and this pathogenic RNA induces human RPE cytotoxicity and RPE degeneration in mice. Antisense oligonucleotides targeting Alu/B1/B2 RNAs prevent DICER1 depletion-induced RPE degeneration despite global miRNA downregulation. DICER1 degrades Alu RNA, and this digested Alu RNA cannot induce RPE degeneration in mice. These findings reveal a miRNA-independent cell survival function for DICER1 involving retrotransposon transcript degradation, show that Alu RNA can directly cause human pathology, and identify new targets for a major cause of blindness.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Data deposits
The Alu sequences have been deposited in GenBank under the accession numbers HN176584 and HN176585.
Change history
16 March 2011
In the paragraph beginning, ‘Subretinal injection delivered Alu RNA to RPE cells...’, two figure citations were corrected.
References
Kleinman, M. E. et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature 452, 591–597 (2008)
Takeda, A. et al. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature 460, 225–230 (2009)
Ferrara, N. Vascular endothelial growth factor and age-related macular degeneration: from basic science to therapy. Nature Med. 16, 1107–1111 (2010)
Ambati, J., Ambati, B. K., Yoo, S. H., Ianchulev, S. & Adamis, A. P. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv. Ophthalmol. 48, 257–293 (2003)
Bernstein, E., Caudy, A. A., Hammond, S. M. & Hannon, G. J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366 (2001)
Batzer, M. A. & Deininger, P. L. Alu repeats and human genomic diversity. Nature Rev. Genet. 3, 370–379 (2002)
Gregory, R. I. et al. The Microprocessor complex mediates the genesis of microRNAs. Nature 432, 235–240 (2004)
Liu, J. et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science 305, 1437–1441 (2004)
Meister, G. et al. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell 15, 185–197 (2004)
Harfe, B. D., McManus, M. T., Mansfield, J. H., Hornstein, E. & Tabin, C. J. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc. Natl Acad. Sci. USA 102, 10898–10903 (2005)
Iacovelli, J. et al. Generation of cre transgenic mice with postnatal RPE-specific ocular expression. Invest. Ophthalmol. Vis. Sci. 10.1167/iovs.10–6347 (6 January 2011)
Alexander, J. J. & Hauswirth, W. W. Adeno-associated viral vectors and the retina. Adv. Exp. Med. Biol. 613, 121–128 (2008)
Chong, M. M., Rasmussen, J. P., Rudensky, A. Y. & Littman, D. R. The RNAseIII enzyme Drosha is critical in T cells for preventing lethal inflammatory disease. J. Exp. Med. 205, 2005–2017 (2008)
Yi, R. et al. DGCR8-dependent microRNA biogenesis is essential for skin development. Proc. Natl Acad. Sci. USA 106, 498–502 (2009)
O’Carroll, D. et al. A Slicer-independent role for Argonaute 2 in hematopoiesis and the microRNA pathway. Genes Dev. 21, 1999–2004 (2007)
Chong, M. M. et al. Canonical and alternate functions of the microRNA biogenesis machinery. Genes Dev. 24, 1951–1960 (2010)
Babiarz, J. E., Ruby, J. G., Wang, Y., Bartel, D. P. & Blelloch, R. Mouse ES cells express endogenous shRNAs, siRNAs, and other Microprocessor-independent, Dicer-dependent small RNAs. Genes Dev. 22, 2773–2785 (2008)
Schaefer, A. et al. Argonaute 2 in dopamine 2 receptor-expressing neurons regulates cocaine addiction. J. Exp. Med. 207, 1843–1851 (2010)
Diederichs, S. & Haber, D. A. Dual role for Argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell 131, 1097–1108 (2007)
Kaneda, M., Tang, F., O’Carroll, D., Lao, K. & Surani, M. A. Essential role for Argonaute2 protein in mouse oogenesis. Epigenetics Chromatin 2, 9 (2009)
Su, H., Trombly, M. I., Chen, J. & Wang, X. Essential and overlapping functions for mammalian Argonautes in microRNA silencing. Genes Dev. 23, 304–317 (2009)
Chendrimada, T. P. et al. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 436, 740–744 (2005)
Cummins, J. M. et al. The colorectal microRNAome. Proc. Natl Acad. Sci. USA 103, 3687–3692 (2006)
Schonborn, J. et al. Monoclonal antibodies to double-stranded RNA as probes of RNA structure in crude nucleic acid extracts. Nucleic Acids Res. 19, 2993–3000 (1991)
Kato, H. et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 205, 1601–1610 (2008)
Saleh, M. C. et al. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nature Cell Biol. 8, 793–802 (2006)
Yang, Z. et al. Toll-like receptor 3 and geographic atrophy in age-related macular degeneration. N. Engl. J. Med. 359, 1456–1463 (2008)
Dunaief, J. L., Dentchev, T., Ying, G. S. & Milam, A. H. The role of apoptosis in age-related macular degeneration. Arch. Ophthalmol. 120, 1435–1442 (2002)
Davis, T. H. et al. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J. Neurosci. 28, 4322–4330 (2008)
Damiani, D. et al. Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina. J. Neurosci. 28, 4878–4887 (2008)
Chen, J. F. et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc. Natl Acad. Sci. USA 105, 2111–2116 (2008)
Merritt, W. M. et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N. Engl. J. Med. 359, 2641–2650 (2008)
Kumar, M. S. et al. Dicer1 functions as a haploinsufficient tumor suppressor. Genes Dev. 23, 2700–2704 (2009)
Hill, D. A. et al. DICER1 mutations in familial pleuropulmonary blastoma. Science 325, 965 (2009)
Nicholls, R. D., Fischel-Ghodsian, N. & Higgs, D. R. Recombination at the human α-globin gene cluster: sequence features and topological constraints. Cell 49, 369–378 (1987)
Nyström-Lahti, M. et al. Founding mutations and Alu-mediated recombination in hereditary colon cancer. Nature Med. 1, 1203–1206 (1995)
Lehrman, M. A. et al. Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science 227, 140–146 (1985)
Lehrman, M. A., Goldstein, J. L., Russell, D. W. & Brown, M. S. Duplication of seven exons in LDL receptor gene caused by Alu-Alu recombination in a subject with familial hypercholesterolemia. Cell 48, 827–835 (1987)
Wallace, M. R. et al. A de novo Alu insertion results in neurofibromatosis type 1. Nature 353, 864–866 (1991)
Volpe, T. A. et al. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297, 1833–1837 (2002)
Hall, I. M. et al. Establishment and maintenance of a heterochromatin domain. Science 297, 2232–2237 (2002)
Prades, C., Laurent, A. M., Puechberty, J., Yurov, Y. & Roizes, G. SINE and LINE within human centromeres. J. Mol. Evol. 42, 37–43 (1996)
Saito, Y. et al. Chromatin remodeling at Alu repeats by epigenetic treatment activates silenced microRNA-512–5p with downregulation of Mcl-1 in human gastric cancer cells. Oncogene 28, 2738–2744 (2009)
Murchison, E. P., Partridge, J. F., Tam, O. H., Cheloufi, S. & Hannon, G. J. Characterization of Dicer-deficient murine embryonic stem cells. Proc. Natl Acad. Sci. USA 102, 12135–12140 (2005)
Kanellopoulou, C. et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 19, 489–501 (2005)
Tam, O. H. et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature 453, 534–538 (2008)
Watanabe, T. et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature 453, 539–543 (2008)
Nakagawa, A., Shi, Y., Kage-Nakadai, E., Mitani, S. & Xue, D. Caspase-dependent conversion of Dicer ribonuclease into a death-promoting deoxyribonuclease. Science 328, 327–334 (2010)
Kohany, O., Gentles, A. J., Hankus, L. & Jurka, J. Annotation, submission and screening of repetitive elements in Repbase: RepbaseSubmitter and Censor. BMC Bioinformatics 7, 474 (2006)
Yang, P., Tyrrell, J., Han, I. & Jaffe, G. J. Expression and modulation of RPE cell membrane complement regulatory proteins. Invest. Ophthalmol. Vis. Sci. 50, 3473–3481 (2009)
Shaikh, T. H., Roy, A. M., Kim, J., Batzer, M. A. & Deininger, P. L. cDNAs derived from primary and small cytoplasmic Alu (scAlu) transcripts. J. Mol. Biol. 271, 222–234 (1997)
Allen, T. A., Von Kaenel, S., Goodrich, J. A. & Kugel, J. F. The SINE-encoded mouse B2 RNA represses mRNA transcription in response to heat shock. Nature Struct. Mol. Biol. 11, 816–821 (2004)
Acknowledgements
We thank M. Chrenek, J. Garcia-Perez, T. Heidmann, C. Kanellopoulou, D. M. Livingston, J. V. Moran, R. F. Mullins, J. M. Nickerson, E. A. Pearce, A. Tarakhovsky, B. Vogelstein, V. E. Velculescu and D. J. Zack for providing mice, reagents or tissues; R. King, L. Xu, M. McConnell, C. Payne, G. R. Pattison, G. J. Jaffe, S. Medearis and C. Spee for technical assistance; and A. Sinai, R. Mohan, T. S. Khurana, R. A. Brekken, P. L. Deininger, S. Bondada, P. A. Pearson, A. M. Rao, G. S. Rao and K. Ambati for discussions. J.A. was supported by National Eye Institute (NEI)/National Institutes of Health (NIH) grants R01EY015422, R01EY018350, R01EY018836, R01EY020672, R21EY019778, RC1EY020442, the Doris Duke Distinguished Clinical Scientist Award, the Burroughs Wellcome Fund Clinical Scientist Award in Translational Research, and the Dr E. Vernon Smith and Eloise C. Smith Macular Degeneration Endowed Chair. Research to Prevent Blindness Senior Scientist Investigator Awards or departmental unrestricted grants supported J.A., H.E.G. and W.W.H.; J.Z.B. was supported by University of Kentucky Physician Scientist Award, International Retinal Research Foundation, and American Health Assistance Foundation; B.K.A. by VA Merit Award and Department of Defense; D.-k.L. by Global Research Laboratory program by MEST, Korea; D.R.H. by Arnold and Mabel Beckman Foundation; W.W.H. by Macular Vision Research Foundation and Foundation Fighting Blindness; J.M.P. by ARC Centres of Excellence Grant CE0561903; M.C.M. by Sydney Foundation for Medical Research. B.K.A was supported by NIH R01EY017182 and R01EY017950; R.E.B. by NIH R01HD027215; G.C. by NIH R21AI076757; H.E.G. by NIH P30EY06360; W.W.H. by NIH U10EY013729, R01EY011123, and P30EY008571; J.F.K. and J.A.G. by NIH R01GM068414; J.L.D. by NIH R01EY015240; D.R.H. by NIH P30EY003040 and R01EY001545; M.E.K. and S.B. by NIH T32HL091812. P.P. is a Senior Scholar from the Fonds de la Recherche en Santé du Québec (FRSQ). M.M.W.C. is a QEII Fellow of the Australian Research Council and is supported by National Health and Medical Research Council, Australia Project Grant 637228. E.F. and D.R.L. are investigators of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
H.K., S.D., V.T., W.G.C., B.J.F., M.E.K., S.L.P., J.F.K., J.A.G., K.K., N.L.J., B.D.G., Y.H., R.J.C.A., A.D.B., S.B, J.W., M.H, Y.S. and J.Z.B. performed experiments. W.W.H., V.A.C, D.-k.L., J.W.Y., C.M.R, D.R.H., H.E.G., Q.Z., J.M.P., M.C.M., A.H.M., M.M.W.C., D.R.L., E.F., P.P., F.W.B., R.E.B., S.M., G.C. and J.L.D. provided tissues or reagents. J.A. conceived and directed the project, and wrote the paper with assistance from P.P., C.M.R., K.K., J.F.K., J.A.G., E.F., M.M.W.C., B.J.F., B.D.G. and B.K.A. All authors had the opportunity to discuss the results and comment on the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.A. is named as an inventor on patent applications filed by his employer, the University of Kentucky, on technologies described in this paper. W.W.H. and the University of Florida have a financial interest in the use of AAV therapies, and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-26 with legends, Supplementary Notes, supplementary Materials and Methods and additional references. (PDF 2605 kb)
Rights and permissions
About this article
Cite this article
Kaneko, H., Dridi, S., Tarallo, V. et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature 471, 325–330 (2011). https://doi.org/10.1038/nature09830
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09830
This article is cited by
-
Selection on synonymous sites: the unwanted transcript hypothesis
Nature Reviews Genetics (2024)
-
Emerging role of the RNA-editing enzyme ADAR1 in stem cell fate and function
Biomarker Research (2023)
-
cGAS inhibition alleviates Alu RNA-induced immune responses and cytotoxicity in retinal pigmented epithelium
Cell & Bioscience (2022)
-
Cellular origins of dsRNA, their recognition and consequences
Nature Reviews Molecular Cell Biology (2022)
-
Subretinal injection in mice to study retinal physiology and disease
Nature Protocols (2022)