Abstract
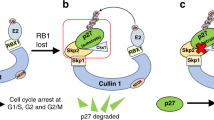
Cellular senescence has been recently shown to have an important role in opposing tumour initiation and promotion. Senescence induced by oncogenes or by loss of tumour suppressor genes is thought to critically depend on induction of the p19Arf–p53 pathway. The Skp2 E3-ubiquitin ligase can act as a proto-oncogene and its aberrant overexpression is frequently observed in human cancers. Here we show that although Skp2 inactivation on its own does not induce cellular senescence, aberrant proto-oncogenic signals as well as inactivation of tumour suppressor genes do trigger a potent, tumour-suppressive senescence response in mice and cells devoid of Skp2. Notably, Skp2 inactivation and oncogenic-stress-driven senescence neither elicit activation of the p19Arf–p53 pathway nor DNA damage, but instead depend on Atf4, p27 and p21. We further demonstrate that genetic Skp2 inactivation evokes cellular senescence even in oncogenic conditions in which the p19Arf–p53 response is impaired, whereas a Skp2–SCF complex inhibitor can trigger cellular senescence in p53/Pten-deficient cells and tumour regression in preclinical studies. Our findings therefore provide proof-of-principle evidence that pharmacological inhibition of Skp2 may represent a general approach for cancer prevention and therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sharpless, N. E. & DePinho, R. A. Cancer: crime and punishment. Nature 436, 636–637 (2005)
Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 (2005)
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007)
Ventura, A. et al. Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661–665 (2007)
Braig, M. et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436, 660–665 (2005)
Michaloglou, C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005)
Collado, M. et al. Tumour biology: senescence in premalignant tumours. Nature 436, 642 (2005)
Cantley, L. C. & Neel, B. G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl Acad. Sci. USA 96, 4240–4245 (1999)
Di Cristofano, A. & Pandolfi, P. P. The multiple roles of PTEN in tumor suppression. Cell 100, 387–390 (2000)
Salmena, L., Carracedo, A. & Pandolfi, P. P. Tenets of PTEN tumor suppression. Cell 133, 403–414 (2008)
Bloom, J. & Pagano, M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin. Cancer Biol. 13, 41–47 (2003)
Nakayama, K. I. & Nakayama, K. Regulation of the cell cycle by SCF-type ubiquitin ligases. Semin. Cell Dev. Biol. 16, 323–333 (2005)
Gstaiger, M. et al. Skp2 is oncogenic and overexpressed in human cancers. Proc. Natl Acad. Sci. USA 98, 5043–5048 (2001)
Latres, E. et al. Role of the F-box protein Skp2 in lymphomagenesis. Proc. Natl Acad. Sci. USA 98, 2515–2520 (2001)
Shim, E. H. et al. Expression of the F-box protein SKP2 induces hyperplasia, dysplasia, and low-grade carcinoma in the mouse prostate. Cancer Res. 63, 1583–1588 (2003)
Lin, H. K. et al. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nature Cell Biol. 11, 420–432 (2009)
Chiarle, R. et al. S-phase kinase-associated protein 2 expression in non-Hodgkin’s lymphoma inversely correlates with p27 expression and defines cells in S phase. Am. J. Pathol. 160, 1457–1466 (2002)
Nakayama, K. et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploidy and centrosome overduplication. EMBO J. 19, 2069–2081 (2000)
Lin, A. W. et al. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12, 3008–3019 (1998)
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997)
Kim, W. Y. & Sharpless, N. E. The regulation of INK4/ARF in cancer and aging. Cell 127, 265–275 (2006)
Yaswen, P. & Campisi, J. Oncogene-induced senescence pathways weave an intricate tapestry. Cell 128, 233–234 (2007)
Hemann, M. T. et al. An epi-allelic series of p53 hypomorphs created by stable RNAi produces distinct tumor phenotypes in vivo. Nature Genet. 33, 396–400 (2003)
Gottlieb, E., Haffner, R., von Ruden, T., Wagner, E. F. & Oren, M. Down-regulation of wild-type p53 activity interferes with apoptosis of IL-3-dependent hematopoietic cells following IL-3 withdrawal. EMBO J. 13, 1368–1374 (1994)
Chen, Z. et al. Differential p53-independent outcomes of p19Arf loss in oncogenesis. Sci. Signal. 2, ra44 (2009)
Bartkova, J. et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444, 633–637 (2006)
Di Micco, R. et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444, 638–642 (2006)
Mallette, F. A., Gaumont-Leclerc, M. F. & Ferbeyre, G. The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Dev. 21, 43–48 (2007)
Yu, Z. K., Gervais, J. L. & Zhang, H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21CIP1/WAF1and cyclin D proteins. Proc. Natl Acad. Sci. USA 95, 11324–11329 (1998)
Denoyelle, C. et al. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nature Cell Biol. 8, 1053–1063 (2006)
Kim, I., Xu, W. & Reed, J. C. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nature Rev. Drug Discov. 7, 1013–1030 (2008)
Di Cristofano, A. et al. Impaired Fas response and autoimmunity in Pten+/- mice. Science 285, 2122–2125 (1999)
Di Cristofano, A., Pesce, B., Cordon-Cardo, C. & Pandolfi, P. P. Pten is essential for embryonic development and tumour suppression. Nature Genet. 19, 348–355 (1998)
Kamijo, T., Bodner, S., van de Kamp, E., Randle, D. H. & Sherr, C. J. Tumor spectrum in ARF-deficient mice. Cancer Res. 59, 2217–2222 (1999)
Kamijo, T. et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91, 649–659 (1997)
Soucy, T. A. et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458, 732–736 (2009)
Young, A. P. et al. VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nature Cell Biol. 10, 361–369 (2008)
Kuo, Y. L. & Giam, C. Z. Activation of the anaphase promoting complex by HTLV-1 tax leads to senescence. EMBO J. 25, 1741–1752 (2006)
Agarwal, A. et al. Absence of SKP2 expression attenuates BCR-ABL-induced myeloproliferative disease. Blood 112, 1960–1970 (2008)
Lin, H. K., Bergmann, S. & Pandolfi, P. P. Cytoplasmic PML function in TGF-β signalling. Nature 431, 205–211 (2004)
Yang, W. L. et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science 325, 1134–1138 (2009)
Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 (2005)
Acknowledgements
We are grateful to C. J. Sherr, S. W. Lowe and M. Oren for mice and reagents. We would also like to thank B. Carver, L. DiSantis, J. Clossey and S. Megan for editing and critical reading of the manuscript, J. A. Koutcher, C. Le, C. Matei and M. Lupa for MRI analysis, as well all the members of the Pandolfi laboratory for comments and discussion. We extend our thanks to M. Rolfe, P. G. Smith, and Millennium Pharmaceuticals for discussion and for providing the MLN4924 compound. This work was supported by NIH grants to P.P.P. and M.D. Anderson Trust Scholar Award and DOD Prostate Cancer New Investigator Award to H.K.L.
Author Contributions H.K.L. and P.P.P. designed the experiments and wrote the manuscript; H.-K.L., Z.C., G.W., S.-W.L., C.N., C.-H.C., W.-L.Y., J.W. and A.E. performed the experiments; C.C.-C. and J.T-F. performed the histopathological analysis of the mice; K.I.N. provided the Skp2-/- mice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures S1-S17 with legends. (PDF 2787 kb)
Rights and permissions
About this article
Cite this article
Lin, HK., Chen, Z., Wang, G. et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature 464, 374–379 (2010). https://doi.org/10.1038/nature08815
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08815