Abstract
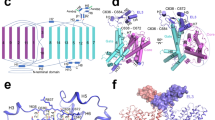
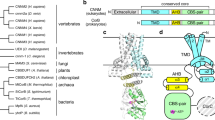
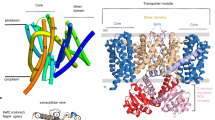
FocA is a representative member of the formate–nitrite transporter family, which transports short-chain acids in bacteria, archaea, fungi, algae and parasites. The structure and transport mechanism of the formate–nitrite transporter family remain unknown. Here we report the crystal structure of Escherichia coli FocA at 2.25 Å resolution. FocA forms a symmetric pentamer, with each protomer consisting of six transmembrane segments. Despite a lack of sequence homology, the overall structure of the FocA protomer closely resembles that of aquaporin and strongly argues that FocA is a channel, rather than a transporter. Structural analysis identifies potentially important channel residues, defines the channel path and reveals two constriction sites. Unlike aquaporin, FocA is impermeable to water but allows the passage of formate. A structural and biochemical investigation provides mechanistic insights into the channel activity of FocA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Stams, A. J. & Plugge, C. M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nature Rev. Microbiol. 7, 568–577 (2009)
White, W. B. & Ferry, J. G. Identification of formate dehydrogenase-specific mRNA species and nucleotide sequence of the fdhC gene of Methanobacterium formicicum . J. Bacteriol. 174, 4997–5004 (1992)
Leonhartsberger, S., Korsa, I. & Bock, A. The molecular biology of formate metabolism in enterobacteria. J. Mol. Microbiol. Biotechnol. 4, 269–276 (2002)
Sawers, R. G. Formate and its role in hydrogen production in Escherichia coli . Biochem. Soc. Trans. 33, 42–46 (2005)
Sawers, G. The hydrogenases and formate dehydrogenases of Escherichia coli . Antonie Van Leeuwenhoek 66, 57–88 (1994)
Stephenson, M. & Stickland, L. H. Hydrogenlyases: bacterial enzymes liberating molecular hydrogen. Biochem. J. 26, 712–724 (1932)
Suppmann, B. & Sawers, G. Isolation and characterization of hypophosphite-resistant mutants of Escherichia coli: identification of the FocA protein, encoded by the pfl operon, as a putative formate transporter. Mol. Microbiol. 11, 965–982 (1994)
Saier, M. H. et al. Phylogenetic characterization of novel transport protein families revealed by genome analyses. Biochim. Biophys. Acta 1422, 1–56 (1999)
Jia, W. & Cole, J. A. Nitrate and nitrite transport in Escherichia coli . Biochem. Soc. Trans. 33, 159–161 (2005)
Jia, W., Tovell, N., Clegg, S., Trimmer, M. & Cole, J. A single channel for nitrate uptake, nitrite export and nitrite uptake by Escherichia coli NarU and a role for NirC in nitrite export and uptake. Biochem. J. 417, 297–304 (2009)
Clegg, S., Yu, F., Griffiths, L. & Cole, J. A. The roles of the polytopic membrane proteins NarK, NarU and NirC in Escherichia coli K-12: two nitrate and three nitrite transporters. Mol. Microbiol. 44, 143–155 (2002)
Clegg, S. J., Jia, W. & Cole, J. A. Role of the Escherichia coli nitrate transport protein, NarU, in survival during severe nutrient starvation and slow growth. Microbiology 152, 2091–2100 (2006)
Nolling, J. & Reeve, J. N. Growth- and substrate-dependent transcription of the formate dehydrogenase (fdhCAB) operon in Methanobacterium thermoformicicum Z-245. J. Bacteriol. 179, 899–908 (1997)
von Heijne, G. & Gavel, Y. Topogenic signals in integral membrane proteins. Eur. J. Biochem. 174, 671–678 (1988)
Smart, O. S., Goodfellow, J. M. & Wallace, B. A. The pore dimensions of gramicidin A. Biophys. J. 65, 2455–2460 (1993)
Fu, D. et al. Structure of a glycerol-conducting channel and the basis for its selectivity. Science 290, 481–486 (2000)
Agre, P. The aquaporin water channels. Proc. Am. Thorac. Soc. 3, 5–13 (2006)
Gonen, T. & Walz, T. The structure of aquaporins. Q. Rev. Biophys. 39, 361–396 (2006)
Stroud, R. M., Nollert, P. & Miercke, L. The glycerol facilitator GlpF its aquaporin family of channels, and their selectivity. Adv. Protein Chem. 63, 291–316 (2003)
Carbrey, J. M. & Agre, P. Discovery of the aquaporins and development of the field. Handb. Exp. Pharmacol. 190, 3–28 (2009)
Holm, L. & Sander, C. Protein structure comparison by alignment of distance matrices. J. Mol. Biol. 233, 123–138 (1993)
Savage, D. F., Egea, P. F., Robles-Colmenares, Y., O’Connell, J. D. & Stroud, R. M. Architecture and selectivity in aquaporins: 2.5 Å X-ray structure of aquaporin Z. PLoS Biol. 1, E72 (2003)
Sui, H., Han, B. G., Lee, J. K., Walian, P. & Jap, B. K. Structural basis of water-specific transport through the AQP1 water channel. Nature 414, 872–878 (2001)
Murata, K. et al. Structural determinants of water permeation through aquaporin-1. Nature 407, 599–605 (2000)
Tajkhorshid, E. et al. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science 296, 525–530 (2002)
Gonen, T. et al. Lipid–protein interactions in double-layered two-dimensional AQP0 crystals. Nature 438, 633–638 (2005)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Collaborative Computational Project Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Schneider, T. R. & Sheldrick, G. M. Substructure solution with SHELXD. Acta Crystallogr. D 58, 1772–1779 (2002)
Cowtan, K. dm: an automated procedure for phase improvement by density modification. Joint CCP4 and ESF-EACBM Newsl. Protein Crystallogr. 31, 34–38 (1994)
Cowtan, K. The Buccaneer software for automated model building. Acta Crystallogr. D 62, 1002–1011 (2006)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D 58, 1948–1954 (2002)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Cryst. 40, 658–674 (2007)
Borgnia, M. J., Kozono, D., Calamita, G., Maloney, P. C. & Agre, P. Functional reconstitution and characterization of AqpZ, the E. coli water channel protein. J. Mol. Biol. 291, 1169–1179 (1999)
DeLano, W. L. PyMOL Molecular Viewer. http://www.pymol.org (2002)
Borgnia, M. J. & Agre, P. Reconstitution and functional comparison of purified GlpF and AqpZ, the glycerol and water channels from Escherichia coli . Proc. Natl Acad. Sci. USA 98, 2888–2893 (2001)
Khademi, S. et al. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 Å. Science 305, 1587–1594 (2004)
Acknowledgements
We thank N. Shimizu, S. Baba and T. Kumasaka at the Spring-8 beamline BL41XU for assistance, and J. He and S. Huang for help at Shanghai Synchrotron Radiation Facility (SSRF). This work was supported by funds from the Ministry of Science and Technology of China (grants 2009CB918801 and 2009CB918802), Tsinghua University 985 Phase II funds, Project 30888001 supported by National Natural Science Foundation of China, and the Beijing Municipal Commissions of Education and Science and Technology. N.Y. acknowledges support from the Yuyuan Foundation and Li’s Foundation.
Author Contributions Experiments were performed by Y.W., Y.H., J.W., C.C., W.H., P.L., Y.-N.X., P.W. and N.Y. Data were analysed by Y.W., Y.H., J.W., N.Y. and Y.S. The manuscript was prepared by N.Y. and Y.S.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
This file contains Supplementary Table 1 and Supplementary Figures 1- 11 with Legends. (PDF 1064 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Huang, Y., Wang, J. et al. Structure of the formate transporter FocA reveals a pentameric aquaporin-like channel . Nature 462, 467–472 (2009). https://doi.org/10.1038/nature08610
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08610
This article is cited by
-
Comparison of ion selectivities of nitrite channel NirC and water channel aquaporin
World Journal of Microbiology and Biotechnology (2023)
-
Achieving high titer and yield in the bioconversion of l-threonine to 2-hydroxybutyric acid with Escherichia coli BL21
Systems Microbiology and Biomanufacturing (2023)
-
Bioconversion of CO to formate by artificially designed carbon monoxide:formate oxidoreductase in hyperthermophilic archaea
Communications Biology (2022)
-
H2-dependent formate production by hyperthermophilic Thermococcales: an alternative to sulfur reduction for reducing-equivalents disposal
The ISME Journal (2021)
-
Potential virus-mediated nitrogen cycling in oxygen-depleted oceanic waters
The ISME Journal (2021)