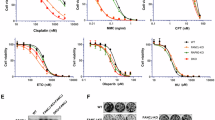
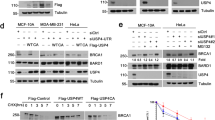
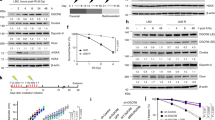
Abstract
Mutations in BRCA1 are associated with a high risk of breast and ovarian cancer. BRCA1 participates in the DNA damage response and acts as a ubiquitin ligase. However, its regulation remains poorly understood. Here we report that BRCA1 is modified by small ubiquitin-like modifier (SUMO) in response to genotoxic stress, and co-localizes at sites of DNA damage with SUMO1, SUMO2/3 and the SUMO-conjugating enzyme Ubc9. PIAS SUMO E3 ligases co-localize with and modulate SUMO modification of BRCA1, and are required for BRCA1 ubiquitin ligase activity in cells. In vitro SUMO modification of the BRCA1/BARD1 heterodimer greatly increases its ligase activity, identifying it as a SUMO-regulated ubiquitin ligase (SRUbL). Further, PIAS SUMO ligases are required for complete accumulation of double-stranded DNA (dsDNA) damage-repair proteins subsequent to RNF8 accrual, and for proficient double-strand break repair. These data demonstrate that the SUMOylation pathway plays a significant role in mammalian DNA damage response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lorick, K. L. et al. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc. Natl Acad. Sci. USA 96, 11364–11369 (1999)
Hashizume, R. et al. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J. Biol. Chem. 276, 14537–14540 (2001)
Brzovic, P. S. et al. Binding and recognition in the assembly of an active BRCA1/BARD1 ubiquitin-ligase complex. Proc. Natl Acad. Sci. USA 100, 5646–5651 (2003)
Morris, J. R. et al. Genetic analysis of BRCA1 ubiquitin ligase activity and its relationship to breast cancer susceptibility. Hum. Mol. Genet. 15, 599–606 (2006)
Morris, J. R. & Solomon, E. BRCA1: BARD1 induces the formation of conjugated ubiquitin structures, dependent on K6 of ubiquitin, in cells during DNA replication and repair. Hum. Mol. Genet. 13, 807–817 (2004)
Morris, J. R. & Solomon, E. in The Role of Genetics in Breast and Reproductive Cancers (ed. Welcsh, P. L.) 75–92 (Springer Science+Business Media and Humana Press, 2009)
Reid, L. J. et al. E3 ligase activity of BRCA1 is not essential for mammalian cell viability or homology-directed repair of double-strand DNA breaks. Proc. Natl Acad. Sci. USA 105, 20876–20881 (2008)
Polanowska, J., Martin, J. S., Garcia-Muse, T., Petalcorin, M. I. & Boulton, S. J. A conserved pathway to activate BRCA1-dependent ubiquitylation at DNA damage sites. EMBO J. 25, 2178–2188 (2006)
Zhao, G. Y. et al. A critical role for the ubiquitin-conjugating enzyme Ubc13 in initiating homologous recombination. Mol. Cell 25, 663–675 (2007)
Doil, C. et al. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell 136, 435–446 (2009)
Huen, M. S. et al. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell 131, 901–914 (2007)
Kolas, N. K. et al. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science 318, 1637–1640 (2007)
Mailand, N. et al. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell 131, 887–900 (2007)
Wang, B. & Elledge, S. J. Ubc13/Rnf8 ubiquitin ligases control foci formation of the Rap80/Abraxas/Brca1/Brcc36 complex in response to DNA damage. Proc. Natl Acad. Sci. USA 104, 20759–20763 (2007)
Kim, H., Huang, J. & Chen, J. CCDC98 is a BRCA1-BRCT domain-binding protein involved in the DNA damage response. Nature Struct. Mol. Biol. 14, 710–715 (2007)
Kim, H., Chen, J. & Yu, X. Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response. Science 316, 1202–1205 (2007)
Liu, Z., Wu, J. & Yu, X. CCDC98 targets BRCA1 to DNA damage sites. Nature Struct. Mol. Biol. 14, 716–720 (2007)
Sobhian, B. et al. RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science 316, 1198–1202 (2007)
Wang, B. et al. Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science 316, 1194–1198 (2007)
Yan, J. et al. The ubiquitin-interacting motif containing protein RAP80 interacts with BRCA1 and functions in DNA damage repair response. Cancer Res. 67, 6647–6656 (2007)
Stewart, G. S. et al. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell 136, 420–434 (2009)
Hay, R. T. SUMO: a history of modification. Mol. Cell 18, 1–12 (2005)
Mo, Y. Y., Yu, Y., Ee, P. L. & Beck, W. T. Overexpression of a dominant-negative mutant Ubc9 is associated with increased sensitivity to anticancer drugs. Cancer Res. 64, 2793–2798 (2004)
Zhao, X. & Blobel, G. A. SUMO ligase is part of a nuclear multiprotein complex that affects DNA repair and chromosomal organization. Proc. Natl Acad. Sci. USA 102, 4777–4782 (2005)
Potts, P. R. & Yu, H. Human MMS21/NSE2 is a SUMO ligase required for DNA repair. Mol. Cell. Biol. 25, 7021–7032 (2005)
Mabb, A. M., Wuerzberger-Davis, S. M. & Miyamoto, S. PIASy mediates NEMO sumoylation and NF-κB activation in response to genotoxic stress. Nature Cell Biol. 8, 986–993 (2006)
Ishiai, M. et al. DNA cross-link repair protein SNM1A interacts with PIAS1 in nuclear focus formation. Mol. Cell. Biol. 24, 10733–10741 (2004)
Boulton, S. J. et al. BRCA1/BARD1 orthologs required for DNA repair in Caenorhabditis elegans . Curr. Biol. 14, 33–39 (2004)
Park, M. A., Seok, Y. J., Jeong, G. & Lee, J. S. SUMO1 negatively regulates BRCA1-mediated transcription, via modulation of promoter occupancy. Nucleic Acids Res. 36, 263–283 (2008)
Peter, M. & Ameer-Beg, S. M. Imaging molecular interactions by multiphoton FLIM. Biol. Cell 96, 231–236 (2004)
Peter, M. et al. Multiphoton-FLIM quantification of the EGFP-mRFP1 FRET pair for localization of membrane receptor-kinase interactions. Biophys. J. 88, 1224–1237 (2005)
Ng, T. et al. Imaging protein kinase Cα activation in cells. Science 283, 2085–2089 (1999)
Ganesan, S., Ameer-Beg, S. M., Ng, T. T., Vojnovic, B. & Wouters, F. S. A dark yellow fluorescent protein (YFP)-based resonance energy-accepting chromoprotein (REACh) for Forster resonance energy transfer with GFP. Proc. Natl Acad. Sci. USA 103, 4089–4094 (2006)
Dadke, S. et al. Regulation of protein tyrosine phosphatase 1B by sumoylation. Nature Cell Biol. 9, 80–85 (2007)
Bossis, G. & Melchior, F. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol. Cell 21, 349–357 (2006)
Schmidt, D. & Muller, S. PIAS/SUMO: new partners in transcriptional regulation. Cell. Mol. Life Sci. 60, 2561–2574 (2003)
Munarriz, E. et al. PIAS-1 is a checkpoint regulator which affects exit from G1 and G2 by sumoylation of p73. Mol. Cell. Biol. 24, 10593–10610 (2004)
Kim, H. & Chen, J. New players in the BRCA1-mediated DNA damage responsive pathway. Mol. Cells 25, 457–461 (2008)
Nishikawa, H., Ooka, S., Sato, K., Arima, K., Okamoto, J., Klevit, R. E., Fukuda, M. & Ohta, T. Mass spectrometric and mutational analyses reveal Lys-6-linked polyubiquitin chains catalyzed by BRCA1-BARD1 ubiquitin ligase. J Biol Chem (2003)
Wu-Baer, F., Lagrazon, K., Yuan, W. & Baer, R. The BRCA1/BARD1 heterodimer assembles polyubiquitin chains through an unconventional linkage involving lysine residue K6 of ubiquitin. J. Biol. Chem. 278, 34743–34746 (2003)
Mallery, D. L., Vandenberg, C. J. & Hiom, K. Activation of the E3 ligase function of the BRCA1/BARD1 complex by polyubiquitin chains. EMBO J. 21, 6755–6762 (2002)
Christensen, D. E., Brzovic, P. S. & Klevit, R. E. E2-BRCA1 RING interactions dictate synthesis of mono- or specific polyubiquitin chain linkages. Nature Struct. Mol. Biol. 14, 941–948 (2007)
Manke, I. A., Lowery, D. M., Nguyen, A. & Yaffe, M. B. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science 302, 636–639 (2003)
Jaffray, E. G. & Hay, R. T. Detection of modification by ubiquitin-like proteins. Methods 38, 35–38 (2006)
Prag, S. et al. Activated ezrin promotes cell migration through recruitment of the GEF Dbl to lipid rafts and preferential downstream activation of Cdc42. Mol. Biol. Cell 18, 2935–2948 (2007)
Barber, P. R., Ameer-Beg, S. M., Gilbey, J. D., Edens, R. J., Ezike, I. & Vojnovic, B. Global and pixel kinetic data analysis for FRET detection by multi-photon time-domain FLIM. Proc. SPIE 5700, 171–181 (2005)
Bennardo, N., Cheng, A., Huang, N. & Stark, J. M. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet. 4, e1000110 (2008)
Boutell, C., Orr, A. & Everett, R. D. PML residue lysine 160 is required for the degradation of PML induced by herpes simplex virus type 1 regulatory protein ICP0. J. Virol. 77, 8686–8694 (2003)
Boutell, C., Sadis, S. & Everett, R. D. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro . J. Virol. 76, 841–850 (2002)
Bliss, C. I. The toxicity of poisons applied jointly. Ann. Appl. Biol. 26, 585–615 (1939)
Acknowledgements
We are grateful to V. De Laurenzi for the PIAS1 expression constructs, to R. Hay for His-SUMO1 and His-SUMO2 cells, to D. Durocher for anti-RNF168 antibody, to G. Stewart for discussions and S. Jackson for sharing results before publication. The work was supported by grants from Breast Cancer Campaign (to J.R.M., A.A., #SF06, and L.B., #06NovPHD13Morris), Cancer Research UK (to R.D., #C8820/A9494), the Medical Research Council (to E.S. and D.W., #6900577, and C.B.), the Richard Dimbleby Cancer Fund to King’s College London (to M.K. and T.N.) and Breakthrough Breast Cancer (to T.K). Multiphoton FLIM systems and acquisition/analysis software were built by S. Ameer-Beg, P. Barber and B. Vojnovic, with support from MRC Co-operative Group Grant G0100152 #56891 and UK Research Councils Basic Technology Research Programme Grant GR/R87901/01.
Author Contributions J.R.M. conceived and designed the study, generated reagents, performed experiments and wrote the paper. C.B. performed in vitro assays, confirmed and developed the initial concept, and generated reagents. M.K. optimised and performed FLIM measurements and analysis. R.D. and D.W. performed experiments and generated reagents. L.B. performed co-localisation observations, A.A. and L.P. generated reagents and Y.G. generated reagents and participated in discussions. T.K. undertook FLIM measurements. T.N. provided expertise and input into the design of the FLIM experiments, and E.S. provided advice and mentoring to J.R.M.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-5 with Legends. (PDF 3343 kb)
Rights and permissions
About this article
Cite this article
Morris, J., Boutell, C., Keppler, M. et al. The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature 462, 886–890 (2009). https://doi.org/10.1038/nature08593
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08593
This article is cited by
-
SENP6 regulates localization and nuclear condensation of DNA damage response proteins by group deSUMOylation
Nature Communications (2023)
-
The SUMOylation and ubiquitination crosstalk in cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Crosstalk between SUMOylation and ubiquitylation controls DNA end resection by maintaining MRE11 homeostasis on chromatin
Nature Communications (2022)
-
PRMT1 and PRMT5: on the road of homologous recombination and non-homologous end joining
Genome Instability & Disease (2022)
-
RNF168-mediated localization of BARD1 recruits the BRCA1-PALB2 complex to DNA damage
Nature Communications (2021)