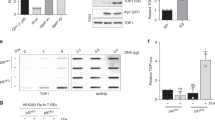
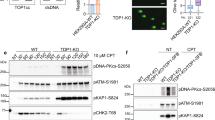
Abstract
Topoisomerases regulate DNA topology and are fundamental to many aspects of chromosome metabolism1,2. Their activity involves the transient cleavage of DNA, which, if it occurs near sites of endogenous DNA damage or in the presence of topoisomerase poisons, can result in abortive topoisomerase-induced DNA strand breaks3,4,5. These breaks feature covalent linkage of the enzyme to the DNA termini by a 3′- or 5′-phosphotyrosyl bond and are implicated in hereditary human disease6,7,8, chromosomal instability and cancer4,9, and underlie the clinical efficacy of an important class of anti-tumour poisons3,9,10. The importance of liberating DNA termini from trapped topoisomerase is illustrated by the progressive neurodegenerative disease observed in individuals containing a mutation in tyrosyl-DNA phosphodiesterase 1 (TDP1), an enzyme that cleaves 3′-phosphotyrosyl bonds6,7,8. However, a complementary human enzyme that cleaves 5′-phosphotyrosyl bonds has not been reported, despite the effect of DNA double-strand breaks containing such termini on chromosome instability and cancer6,7,8. Here we identify such an enzyme in human cells and show that this activity efficiently restores 5′-phosphate termini at DNA double-strand breaks in preparation for DNA ligation. This enzyme, TTRAP, is a member of the Mg2+/Mn2+-dependent family of phosphodiesterases. Cellular depletion of TTRAP results in increased susceptibility and sensitivity to topoisomerase-II-induced DNA double-strand breaks. TTRAP is, to our knowledge, the first human 5′-tyrosyl DNA phosphodiesterase to be identified, and we suggest that this enzyme is denoted tyrosyl DNA phosphodiesterase-2 (TDP2).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Champoux, J. J. DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem. 70, 369–413 (2001)
Wang, J. C. Cellular roles of DNA topoisomerases: a molecular perspective. Nature Rev. Mol. Cell Biol. 3, 430–440 (2002)
Li, T. K. & Liu, L. F. Tumor cell death induced by topoisomerase-targeting drugs. Annu. Rev. Pharmacol. Toxicol. 41, 53–77 (2001)
Deweese, J. E. & Osheroff, N. The DNA cleavage reaction of topoisomerase II: wolf in sheep’s clothing. Nucleic Acids Res. 37, 738–748 (2009)
Pourquier, P. & Pommier, Y. Topoisomerase I-mediated DNA damage. Adv. Cancer Res. 80, 189–216 (2001)
El-Khamisy, S. F. et al. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature 434, 108–113 (2005)
Takashima, H. et al. Mutation of TDP1, encoding a topoisomerase I-dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nature Genet. 32, 267–272 (2002)
Yang, S. W. et al. A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type I topoisomerases. Proc. Natl Acad. Sci. USA 93, 11534–11539 (1996)
Nitiss, J. L. Targeting DNA topoisomerase II in cancer chemotherapy. Nature Rev. Cancer 9, 338–350 (2009)
Pommier, Y. Topoisomerase I inhibitors: camptothecins and beyond. Nature Rev. Cancer 6, 789–802 (2006)
Vance, J. R. & Wilson, T. E. Yeast Tdp1 and Rad1-Rad10 function as redundant pathways for repairing Top1 replicative damage. Proc. Natl Acad. Sci. USA 99, 13669–13674 (2002)
Liu, C., Pouliot, J. J. & Nash, H. A. Repair of topoisomerase I covalent complexes in the absence of the tyrosyl-DNA phosphodiesterase Tdp1. Proc. Natl Acad. Sci. USA 99, 14970–14975 (2002)
Pype, S. et al. TTRAP, a novel protein that associates with CD40, tumor necrosis factor (TNF) receptor-75 and TNF receptor-associated factors (TRAFs), and that inhibits nuclear factor-κB activation. J. Biol. Chem. 275, 18586–18593 (2000)
Rodrigues-Lima, F., Josephs, M., Katan, M. & Cassinat, B. Sequence analysis identifies TTRAP, a protein that associates with CD40 and TNF receptor-associated factors, as a member of a superfamily of divalent cation-dependent phosphodiesterases. Biochem. Biophys. Res. Commun. 285, 1274–1279 (2001)
Nitiss, J. L. et al. Amsacrine and etoposide hypersensitivity of yeast cells overexpressing DNA topoisomerase II. Cancer Res. 52, 4467–4472 (1992)
Xu, G. L. et al. TTRAP is a novel PML nuclear bodies-associated protein. Biochem. Biophys. Res. Commun. 375, 395–398 (2008)
Mielke, C., Christensen, M. O., Barthelmes, H. U. & Boege, F. Enhanced processing of UVA-irradiated DNA by human topoisomerase II in living cells. J. Biol. Chem. 279, 20559–20562 (2004)
Mielke, C., Kalfalah, F. M., Christensen, M. O. & Boege, F. Rapid and prolonged stalling of human DNA topoisomerase I in UVA-irradiated genomic areas. DNA Repair (Amst.) 6, 1757–1763 (2007)
Kingma, P. S. & Osheroff, N. The response of eukaryotic topoisomerases to DNA damage. Biochim. Biophys. Acta 1400, 223–232 (1998)
Connelly, J. C. & Leach, D. R. Repair of DNA covalently linked to protein. Mol. Cell 13, 307–316 (2004)
Hartsuiker, E., Neale, M. J. & Carr, A. M. Distinct requirements for the Rad32(Mre11) nuclease and Ctp1(CtIP) in the removal of covalently bound topoisomerase I and II from DNA. Mol. Cell 33, 117–123 (2009)
Pei, H. et al. EAPII interacts with ETS1 and modulates its transcriptional function. Oncogene 22, 2699–2709 (2003)
Esguerra, C. V. et al. Ttrap is an essential modulator of Smad3-dependent Nodal signaling during zebrafish gastrulation and left-right axis determination. Development 134, 4381–4393 (2007)
Zucchelli, S. et al. Aggresome-forming TTRAP mediates pro-apoptotic properties of Parkinson’s disease-associated DJ-1 missense mutations. Cell Death Differ. 16, 428–438 (2009)
Vance, J. R. & Wilson, T. E. Repair of DNA strand breaks by the overlapping functions of lesion-specific and non-lesion-specific DNA 3′ phosphatases. Mol. Cell. Biol. 21, 7191–7198 (2001)
Nitiss, K. C., Malik, M., He, X., White, S. W. & Nitiss, J. L. Tyrosyl-DNA phosphodiesterase (Tdp1) participates in the repair of Top2-mediated DNA damage. Proc. Natl Acad. Sci. USA 103, 8953–8958 (2006)
Whitehouse, C. J. et al. XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell 104, 107–117 (2001)
Parsons, J. L., Dianova, I. I. & Dianov, G. L. APE1 is the major 3′-phosphoglycolate activity in human cell extracts. Nucleic Acids Res. 32, 3531–3536 (2004)
Reynolds, J. J. et al. Defective DNA ligation during short-patch single-strand break repair in ataxia oculomotor apraxia-1. Mol. Cell. Biol. 29, 1354–1362 (2008)
Katyal, S. et al. TDP1 facilitates chromosomal single-strand break repair in neurons and is neuroprotective in vivo . EMBO J. 26, 4720–4731 (2007)
Acknowledgements
This work was funded by the MRC (G0600776), the BBSRC (BB/C516595/1), and CR-UK (C6563/A10192). S.F.E.K. and F.C.L. were also funded by Fellowships from the Wellcome Trust (S.F.E.K.; 085284), Marie Curie (FCL; 2007-2-1-IEF-221222) and EMBO (FCL; ALTF 956-2006). We thank T. Wilson, H. Nash, M. Neale, J. Nitiss and E. Hoffmann for materials.
Author Contributions F.C.L. developed the genetic screen and conducted the mammalian cell experiments. M.C.Z. and F.C.L. conducted the yeast experiments. K.O. and S.F.E.K. prepared the recombinant proteins, and S.F.E.K. conducted the biochemical experiments. K.W.C., F.C.L. and S.F.E.K. designed and interpreted the experiments. K.W.C. coordinated the project and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-7 with Legends. (PDF 965 kb)
Rights and permissions
About this article
Cite this article
Ledesma, F., El Khamisy, S., Zuma, M. et al. A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature 461, 674–678 (2009). https://doi.org/10.1038/nature08444
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08444