Abstract
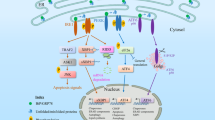
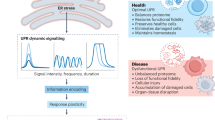
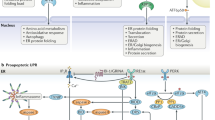
The endoplasmic reticulum is responsible for much of a cell's protein synthesis and folding, but it also has an important role in sensing cellular stress. Recently, it has been shown that the endoplasmic reticulum mediates a specific set of intracellular signalling pathways in response to the accumulation of unfolded or misfolded proteins, and these pathways are collectively known as the unfolded-protein response. New observations suggest that the unfolded-protein response can initiate inflammation, and the coupling of these responses in specialized cells and tissues is now thought to be fundamental in the pathogenesis of inflammatory diseases. The knowledge gained from this emerging field will aid in the development of therapies for modulating cellular stress and inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Charo, I. F. & Ransohoff, R. M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 (2006).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Hansson, G. K. & Libby, P. The immune response in atherosclerosis: a double-edged sword. Nature Rev. Immunol. 6, 508–519 (2006).
Kaufman, R. J. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13, 1211–1233 (1999).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Rev. Mol. Cell Biol. 8, 519–529 (2007).
Schroder, M. & Kaufman, R. J. The mammalian unfolded protein response. Annu. Rev. Biochem. 74, 739–789 (2005).
Mori, K., Ma, W., Gething, M. J. & Sambrook, J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74, 743–756 (1993).
Cox, J. S., Shamu, C. E. & Walter, P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73, 1197–1206 (1993).
Shi, Y. et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 18, 7499–7509 (1998).
Harding, H. P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 (1999).
Haze, K., Yoshida, H., Yanagi, H., Yura, T. & Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787–3799 (1999).
Bertolotti, A., Zhang, Y., Hendershot, L. M., Harding, H. P. & Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nature Cell Biol. 2, 326–332 (2000).
Kohno, K. How transmembrane proteins sense endoplasmic reticulum stress. Antioxid. Redox Signal. 9, 2295–2303 (2007).
Harding, H. P., Zhang, Y., Bertolotti, A., Zeng, H. & Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5, 897–904 (2000).
Lu, P. D., Harding, H. P. & Ron, D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 167, 27–33 (2004).
Yaman, I. et al. The zipper model of translational control: a small upstream ORF is the switch that controls structural remodeling of an mRNA leader. Cell 113, 519–531 (2003).
Harding, H. P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003).
Ye, J. et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6, 1355–1364 (2000).
Yamamoto, K. et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev. Cell 13, 365–376 (2007).
Wu, J. et al. ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell 13, 351–364 (2007).
Ohoka, N., Yoshii, S., Hattori, T., Onozaki, K. & Hayashi, H. TRB3, a novel ER stress-inducible gene, is induced via ATF4–CHOP pathway and is involved in cell death. Embo J. 24, 1243–1255 (2005).
Yamaguchi, H. & Wang, H. G. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 279, 45495–45502 (2004).
Puthalakath, H. et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129, 1337–1349 (2007).
Song, B., Scheuner, D., Ron, D., Pennathur, S. & Kaufman, R. Genetic deletion of C/EBP homologous protein CHOP reduces oxidative stress, improves β cell function, and prevents diabetes. J. Clin. Invest. (in the press). This report describes how the ER-stress-induced pro-apoptotic factor CHOP is involved in oxidative stress and β-cell death.
Raha, S. & Robinson, B. H. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci. 25, 502–508 (2000).
Tu, B. P. & Weissman, J. S. Oxidative protein folding in eukaryotes: mechanisms and consequences. J. Cell Biol. 164, 341–346 (2004).
Tu, B. P. & Weissman, J. S. The FAD- and O2-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol. Cell 10, 983–994 (2002).
Cuozzo, J. W. & Kaiser, C. A. Competition between glutathione and protein thiols for disulphide-bond formation. Nature Cell Biol. 1, 130–135 (1999). References 27 and 28 provide insights into how protein folding in the ER leads to the production of ROS.
Cullinan, S. B. et al. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 23, 7198–7209 (2003).
Mathers, J. et al. Antioxidant and cytoprotective responses to redox stress. Biochem. Soc. Symp. 71, 157–176 (2004).
Zhang, D. D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 38, 769–789 (2006).
Cullinan, S. B. & Diehl, J. A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 279, 20108–20117 (2004).
Rius, J. et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature 453, 807–811 (2008).
Pahl, H. L. & Baeuerle, P. A. Expression of influenza virus hemagglutinin activates transcription factor NF-κB. J. Virol. 69, 1480–1484 (1995).
Meyer, M. et al. Hepatitis B virus transactivator MHBst: activation of NF-κB, selective inhibition by antioxidants and integral membrane localization. Embo J. 11, 2991–3001 (1992).
Pahl, H. L. & Baeuerle, P. A. Activation of NF-κB by ER stress requires both Ca2+ and reactive oxygen intermediates as messengers. FEBS Lett. 392, 129–136 (1996).
Deniaud, A. et al. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene 27, 285–299 (2008). This paper shows that protein misfolding in the ER causes calcium to leak into the cytosol, resulting in the outer membrane of mitochondria becoming more permeable.
Deng, J. et al. Translational repression mediates activation of nuclear factor κB by phosphorylated translation initiation factor 2. Mol. Cell. Biol. 24, 10161–10168 (2004).
Wu, S. et al. Ultraviolet light activates NFκB through translational inhibition of IκBα synthesis. J. Biol. Chem. 279, 34898–34902 (2004). References 38 and 39 show that NF-κB is activated by the PERK pathway of the UPR.
Urano, F. et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 (2000). This paper shows how ER stress activates JNK by way of IRE1α.
Hu, P., Han, Z., Couvillon, A. D., Kaufman, R. J. & Exton, J. H. Autocrine tumor necrosis factor α links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 26, 3071–3084 (2006).
Davis, R. J. Signal transduction by the JNK group of MAP kinases. Cell 103, 239–252 (2000).
Brown, M. S., Ye, J., Rawson, R. B. & Goldstein, J. L. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell 100, 391–398 (2000).
Zhang, K. et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124, 587–599 (2006). This study identifies CREBH, an ER-stress-inducible transcription factor that can mediate the acute-phase response.
Berridge, M. J., Bootman, M. D. & Roderick, H. L. Calcium signalling: dynamics, homeostasis and remodelling. Nature Rev. Mol. Cell Biol. 4, 517–529 (2003).
Gorlach, A., Klappa, P. & Kietzmann, T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid. Redox Signal. 8, 1391–1418 (2006).
Malhotra, J. D. & Kaufman, R. J. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 9, 2277–2293 (2007).
Stamler, J. S., Singel, D. J. & Loscalzo, J. Biochemistry of nitric oxide and its redox-activated forms. Science 258, 1898–1902 (1992).
Uehara, T. et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441, 513–517 (2006).
Xu, K. Y., Huso, D. L., Dawson, T. M., Bredt, D. S. & Becker, L. C. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc. Natl Acad. Sci. USA 96, 657–662 (1999).
Xu, W., Liu, L., Charles, I. G. & Moncada, S. Nitric oxide induces coupling of mitochondrial signalling with the endoplasmic reticulum stress response. Nature Cell Biol. 6, 1129–1134 (2004).
Xue, X. et al. Tumor necrosis factor α (TNFα) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFα. J. Biol. Chem. 280, 33917–33925 (2005).
Lin, W., Harding, H. P., Ron, D. & Popko, B. Endoplasmic reticulum stress modulates the response of myelinating oligodendrocytes to the immune cytokine interferon-γ. J. Cell Biol. 169, 603–612 (2005).
Feng, B. et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nature Cell Biol. 5, 781–792 (2003).
Maedler, K. et al. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J. Clin. Invest. 110, 851–860 (2002).
Kharroubi, I. et al. Free fatty acids and cytokines induce pancreatic β-cell apoptosis by different mechanisms: role of nuclear factor-κB and endoplasmic reticulum stress. Endocrinology 145, 5087–5096 (2004).
Zhou, J. et al. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemic apolipoprotein E-deficient mice. Circulation 110, 207–213 (2004).
Yamamuro, A., Yoshioka, Y., Ogita, K. & Maeda, S. Involvement of endoplasmic reticulum stress on the cell death induced by 6-hydroxydopamine in human neuroblastoma SH-SY5Y cells. Neurochem. Res. 31, 657–664 (2006).
Kaufman, R. J. Orchestrating the unfolded protein response in health and disease. J. Clin. Invest. 110, 1389–1398 (2002).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Ozcan, U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006). This paper shows that decreasing ER stress improves insulin sensitivity in mice with type 2 diabetes.
Hirosumi, J. et al. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002).
Aguirre, V. et al. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 277, 1531–1537 (2002).
Tuncman, G. et al. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc. Natl Acad. Sci. USA 103, 10741–10746 (2006).
Williams, K. J. & Tabas, I. Atherosclerosis and inflammation. Science 297, 521–522 (2002).
Li, Y. et al. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-α and interleukin-6: model of NF-κB- and MAP kinase-dependent inflammation in advanced atherosclerosis. J. Biol. Chem. 280, 21763–21772 (2005). This paper describes how ER-stress signalling and inflammatory-response signalling are integrated in cholesterol-loaded macrophages.
Gargalovic, P. S. et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 26, 2490–2496 (2006).
Tansey, M. G., McCoy, M. K. & Frank-Cannon, T. C. Neuroinflammatory mechanisms in Parkinson's disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp. Neurol. 208, 1–25 (2007).
Lindholm, D., Wootz, H. & Korhonen, L. ER stress and neurodegenerative diseases. Cell Death Differ. 13, 385–392 (2006).
Bence, N. F., Sampat, R. M. & Kopito, R. R. Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552–1555 (2001).
Nishitoh, H. et al. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 22, 1451–1464 (2008).
Wang, H. Q. & Takahashi, R. Expanding insights on the involvement of endoplasmic reticulum stress in Parkinson's disease. Antioxid. Redox Signal. 9, 553–561 (2007).
Silva, R. M. et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J. Neurochem. 95, 974–986 (2005).
Hetz, C. et al. Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis. Proc. Natl Acad. Sci. USA 105, 757–762 (2008).
Paschen, W., Aufenberg, C., Hotop, S. & Mengesdorf, T. Transient cerebral ischemia activates processing of xbp1 messenger RNA indicative of endoplasmic reticulum stress. J. Cereb. Blood Flow Metab. 23, 449–461 (2003).
DeLegge, M. H. & Smoke, A. Neurodegeneration and inflammation. Nutr. Clin. Pract. 23, 35–41 (2008).
Frohman, E. M., Racke, M. K. & Raine, C. S. Multiple sclerosis — the plaque and its pathogenesis. N. Engl. J. Med. 354, 942–955 (2006).
Lin, W. et al. Interferon-γ inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain 129, 1306–1318 (2006).
Lin, W. et al. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J. Clin. Invest. 117, 448–456 (2007). This paper shows that IFN-γ can have a detrimental role or a protective role, mediated by the UPR, depending on the stage of multiple sclerosis.
Lees, J. R. & Cross, A. H. A little stress is good: IFN-γ, demyelination, and multiple sclerosis. J. Clin. Invest. 117, 297–299 (2007).
Tabata, Y. et al. Vaticanol B, a resveratrol tetramer, regulates endoplasmic reticulum stress and inflammation. Am. J. Physiol. Cell. Physiol. 293, C411–C418 (2007).
Boyce, M. et al. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307, 935–939 (2005).
Acknowledgements
We thank J. Mitchell for her efforts in preparing the manuscript. We apologize to those whose work could not be cited because of space limitations. K.Z. is supported by a grant from the American Heart Association (0635423Z). R.J.K. is supported by grants from the National Institutes of Health (DK042394, HL052173 and HL057346) and is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reprints and permissions information is available at http://www.nature.com/reprints.
Correspondence should be addressed to R.J.K. (kaufmanr@umich.edu).
Rights and permissions
About this article
Cite this article
Zhang, K., Kaufman, R. From endoplasmic-reticulum stress to the inflammatory response. Nature 454, 455–462 (2008). https://doi.org/10.1038/nature07203
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07203
This article is cited by
-
Hyperphosphorylated Tau Inflicts Intracellular Stress Responses that Are Mitigated by Apomorphine
Molecular Neurobiology (2024)
-
Empagliflozin reduces endoplasmic reticulum stress associated TXNIP/NLRP3 activation in tunicamycin-stimulated aortic endothelial cells
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Nervous System Response to Neurotrauma: A Narrative Review of Cerebrovascular and Cellular Changes After Neurotrauma
Journal of Molecular Neuroscience (2024)
-
CIS deletion by CRISPR/Cas9 enhances human primary natural killer cell functions against allogeneic glioblastoma
Journal of Experimental & Clinical Cancer Research (2023)
-
Transcriptomic analysis of stem cells from chorionic villi uncovers the impact of chromosomes 2, 6 and 22 in the clinical manifestations of Down syndrome
Stem Cell Research & Therapy (2023)