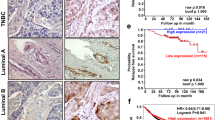
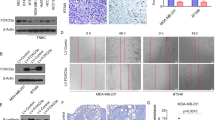
Abstract
MicroRNAs have been implicated in regulating diverse cellular pathways. Although there is emerging evidence that some microRNAs can function as oncogenes or tumour suppressors, the role of microRNAs in mediating cancer metastasis remains unexplored. Here we show, using a combination of mouse and human cells, that microRNA-10b (miR-10b) is highly expressed in metastatic breast cancer cells and positively regulates cell migration and invasion. Overexpression of miR-10b in otherwise non-metastatic breast tumours initiates robust invasion and metastasis. Expression of miR-10b is induced by the transcription factor Twist, which binds directly to the putative promoter of mir-10b (MIRN10B). The miR-10b induced by Twist proceeds to inhibit translation of the messenger RNA encoding homeobox D10, resulting in increased expression of a well-characterized pro-metastatic gene, RHOC. Significantly, the level of miR-10b expression in primary breast carcinomas correlates with clinical progression. These findings suggest the workings of an undescribed regulatory pathway, in which a pleiotropic transcription factor induces expression of a specific microRNA, which suppresses its direct target and in turn activates another pro-metastatic gene, leading to tumour cell invasion and metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Fidler, I. J. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nature Rev. Cancer 3, 453–458 (2003)
Batlle, E. et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nature Cell Biol. 2, 84–89 (2000)
Cano, A. et al. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nature Cell Biol. 2, 76–83 (2000)
Comijn, J. et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol. Cell 7, 1267–1278 (2001)
Bolos, V. et al. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell Sci. 116, 499–511 (2003)
Yang, J. et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 (2004)
Hartwell, K. A. et al. The Spemann organizer gene, Goosecoid, promotes tumor metastasis. Proc. Natl Acad. Sci. USA 103, 18969–18974 (2006)
Mani, S. A. et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc. Natl Acad. Sci. USA 104, 10069–10074 (2007)
Calin, G. A. & Croce, C. M. MicroRNA signatures in human cancers. Nature Rev. Cancer 6, 857–866 (2006)
Esquela-Kerscher, A. & Slack, F. J. Oncomirs — microRNAs with a role in cancer. Nature Rev. Cancer 6, 259–269 (2006)
Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004)
Brennecke, J., Hipfner, D. R., Stark, A., Russell, R. B. & Cohen, S. M. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila . Cell 113, 25–36 (2003)
Chen, C. Z., Li, L., Lodish, H. F. & Bartel, D. P. MicroRNAs modulate hematopoietic lineage differentiation. Science 303, 83–86 (2004)
Poy, M. N. et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 432, 226–230 (2004)
Yi, R. et al. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nature Genet. 38, 356–362 (2006)
Schratt, G. M. et al. A brain-specific microRNA regulates dendritic spine development. Nature 439, 283–289 (2006)
Calin, G. A. et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl Acad. Sci. USA 101, 2999–3004 (2004)
He, L. et al. A microRNA polycistron as a potential human oncogene. Nature 435, 828–833 (2005)
Johnson, S. M. et al. RAS is regulated by the let-7 microRNA family. Cell 120, 635–647 (2005)
Lu, J. et al. MicroRNA expression profiles classify human cancers. Nature 435, 834–838 (2005)
Roldo, C. et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J. Clin. Oncol. 24, 4677–4684 (2006)
Iorio, M. V. et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 65, 7065–7070 (2005)
Meister, G., Landthaler, M., Dorsett, Y. & Tuschl, T. Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA 10, 544–550 (2004)
Cheng, A. M., Byrom, M. W., Shelton, J. & Ford, L. P. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 33, 1290–1297 (2005)
Elenbaas, B. et al. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 15, 50–65 (2001)
Ethier, S. P., Mahacek, M. L., Gullick, W. J., Frank, T. S. & Weber, B. L. Differential isolation of normal luminal mammary epithelial cells and breast cancer cells from primary and metastatic sites using selective media. Cancer Res. 53, 627–635 (1993)
Kuperwasser, C. et al. A mouse model of human breast cancer metastasis to human bone. Cancer Res. 65, 6130–6138 (2005)
Thiery, J. P. Epithelial–mesenchymal transitions in tumour progression. Nature Rev. Cancer 2, 442–454 (2002)
Cripps, R. M. et al. The myogenic regulatory gene Mef2 is a direct target for transcriptional activation by Twist during Drosophila myogenesis. Genes Dev. 12, 422–434 (1998)
Cheng, G. Z. et al. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 67, 1979–1987 (2007)
Zhou, X., Ruan, J., Wang, G. & Zhang, W. Characterization and identification of microRNA core promoters in four model species. PLoS Comput. Biol. 3, e37 (2007)
Lewis, B. P., Shih, I. H., Jones-Rhoades, M. W., Bartel, D. P. & Burge, C. B. Prediction of mammalian microRNA targets. Cell 115, 787–798 (2003)
Krek, A. et al. Combinatorial microRNA target predictions. Nature Genet. 37, 495–500 (2005)
Makiyama, K. et al. Aberrant expression of HOX genes in human invasive breast carcinoma. Oncol. Rep. 13, 673–679 (2005)
Carrio, M., Arderiu, G., Myers, C. & Boudreau, N. J. Homeobox D10 induces phenotypic reversion of breast tumor cells in a three-dimensional culture model. Cancer Res. 65, 7177–7185 (2005)
Myers, C., Charboneau, A., Cheung, I., Hanks, D. & Boudreau, N. Sustained expression of homeobox D10 inhibits angiogenesis. Am. J. Pathol. 161, 2099–2109 (2002)
Clark, E. A., Golub, T. R., Lander, E. S. & Hynes, R. O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406, 532–535 (2000)
Hakem, A. et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 19, 1974–1979 (2005)
Kleer, C. G. et al. RhoC GTPase expression as a potential marker of lymph node metastasis in squamous cell carcinomas of the head and neck. Clin. Cancer Res. 12, 4485–4490 (2006)
Kondo, T. et al. Expression of RhoC is associated with metastasis of gastric carcinomas. Pathobiology 71, 19–25 (2004)
Wang, W. et al. Overexpression of the RhoC gene correlates with invasion and metastasis of hepatocellular carcinoma Chinese J. Oncol . (Zhonghua Zhong Liu Za Zhi.) 26, 279–282 (2004)
Reichmann, E. et al. Activation of an inducible c-FosER fusion protein causes loss of epithelial polarity and triggers epithelial–fibroblastoid cell conversion. Cell 71, 1103–1116 (1992)
Enright, A. J. et al. MicroRNA targets in Drosophila . Genome Biol. 5, R1 (2003)
Yekta, S., Shih, I. H. & Bartel, D. P. MicroRNA-directed cleavage of HOXB8 mRNA. Science 304, 594–596 (2004)
Ronshaugen, M., Biemar, F., Piel, J., Levine, M. & Lai, E. C. The Drosophila microRNA iab-4 causes a dominant homeotic transformation of halteres to wings. Genes Dev. 19, 2947–2952 (2005)
Garzon, R. et al. MicroRNA fingerprints during human megakaryocytopoiesis. Proc. Natl Acad. Sci. USA 103, 5078–5083 (2006)
Zhang, X. et al. Human growth hormone-regulated HOXA1 is a human mammary epithelial oncogene. J. Biol. Chem. 278, 7580–7590 (2003)
Stewart, S. A. et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 9, 493–501 (2003)
Acknowledgements
We thank D. Bartel, H. Lodish, P. Rao, B. Zhou, S. Mani, J. Yang, S. Ethier, C. Largman and L.-H. Wang for reagents and advice; F. Reinhardt for assistance with animal experiments; the Histology Core Laboratory at MIT and MSKCC for assistance with sectioning and immunohistochemistry; C. Mayr, C. Scheel, S. McAllister, I. Ben-Porath, Y. Sun and Y. Luo for critical reading of the manuscript; and members of the Weinberg Laboratory for useful discussions. L.M. is a Susan G. Komen Fellow of the Life Sciences Research Foundation. J.T.-F. is supported by the MSKCC Cancer Core Grant. R.A.W. is an American Cancer Society Research Professor and a Daniel K. Ludwig Cancer Research Professor. This research is supported by an NIH grant (R.A.W.) and the Ludwig Center for Molecular Oncology at MIT.
Author Contributions L.M. conceived the project. R.A.W. supervised research. L.M. designed and performed experiments. L.M. and J.T.-F. collected and analysed data. All authors contributed to the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Methods, Supplementary Figures S1-S2 with Legends and Supplementary Table S1. (PDF 368 kb)
Rights and permissions
About this article
Cite this article
Ma, L., Teruya-Feldstein, J. & Weinberg, R. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 449, 682–688 (2007). https://doi.org/10.1038/nature06174
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06174
This article is cited by
-
Blood-based microRNA profiling unveils complex molecular dynamics in breast cancer
Journal of Applied Genetics (2024)
-
MiRNA-related metastasis in oral cancer: moving and shaking
Cancer Cell International (2023)
-
MicroRNA: trends in clinical trials of cancer diagnosis and therapy strategies
Experimental & Molecular Medicine (2023)
-
Isothermal exponential amplification reactions triggered by circular templates (cEXPAR) targeting miRNA
Molecular Biology Reports (2023)
-
Diagnostic applications and therapeutic option of Cascade CRISPR/Cas in the modulation of miRNA in diverse cancers: promises and obstacles
Journal of Cancer Research and Clinical Oncology (2023)