Abstract
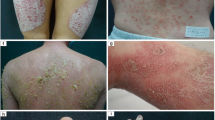
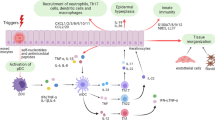
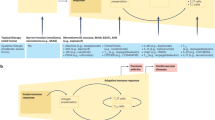
Psoriasis is one of the most common human skin diseases and is considered to have key genetic underpinnings. It is characterized by excessive growth and aberrant differentiation of keratinocytes, but is fully reversible with appropriate therapy. The trigger of the keratinocyte response is thought to be activation of the cellular immune system, with T cells, dendritic cells and various immune-related cytokines and chemokines implicated in pathogenesis. The newest therapies for psoriasis target its immune components and may predict potential treatments for other inflammatory human diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Krueger, J. G. The immunologic basis for the treatment of psoriasis with new biologic agents. J. Am. Acad. Dermatol. 46, 1–23 (2002).
Lebwohl, M. Psoriasis. Lancet 361, 1197–1204 (2003).
Nickoloff, B. J. & Nestle, F. O. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J. Clin. Invest. 113, 1664–1675 (2004).
Bowcock, A. M. & Krueger, J. G. Getting under the skin: the immunogenetics of psoriasis. Nature Rev. Immunol. 5, 699–711 (2005).
Schon, M. P. & Boehncke, W. H. Psoriasis. N. Engl. J. Med. 352, 1899–1912 (2005).
Gaspari, A. A. Innate and adaptive immunity and the pathophysiology of psoriasis. J. Am. Acad. Dermatol. 54, S67–S80 (2006).
Davidson, A. & Diamond, B. Autoimmune diseases. N. Engl. J. Med. 345, 340–350 (2001).
Liu, Y., Krueger, J. G. & Bowcock, A. M. Psoriasis: genetic associations and immune system changes. Genes Immunity advance online publication (9 November 2006) doi:10.1038/sj.gene.6364351.
Christensen, T. E. et al. Observations of psoriasis in the absence of therapeutic intervention identifies two unappreciated morphologic variants, thin-plaque and thick-plaque psoriasis, and their associated phenotypes. J. Invest. Dermatol. 126, 2397–2403 (2006).
Lew, W., Lee, E. & Krueger, J. G. Psoriasis genomics: analysis of proinflammatory (type 1) gene expression in large plaque (Western) and small plaque (Asian) psoriasis vulgaris. Br. J. Dermatol. 150, 668–676 (2004).
Clark, R. A. et al. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 176, 4431–4439 (2006).
Boyman, O. et al. Activation of dendritic antigen-presenting cells expressing common heat shock protein receptor CD91 during induction of psoriasis. Br. J. Dermatol. 152, 1211–1218 (2005).
Boyman, O. et al. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J. Exp. Med. 199, 731–736 (2004).
Lew, W., Bowcock, A. M. & Krueger, J. G. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and 'Type 1' inflammatory gene expression. Trends Immunol. 25, 295–305 (2004).
Nestle, F. O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 202, 135–143 (2005).
Lowes, M. A. et al. Increase in TNF-α and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc. Natl Acad. Sci. USA 102, 19057–19062 (2005).
Larrengina, A. T. & Falo, L. D. Changing paradigms in cutaneous immunology: adapting with dendritic cells. J. Invest. Dermatol. 124, 1–12 (2005).
Serbina, N. V., Salazar-Mather, T. P., Biron, C. A., Kuziel, W. A. & Pamer, E. G. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19, 59–70 (2003).
Lee, E. et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J. Exp. Med. 199, 125–130 (2004).
Wang, F. et al. Prominent production of IL-20 by CD68+/CD11c+ myeloid-derived cells in psoriasis: gene regulation and cellular effects. J. Invest. Dermatol. 126, 1590–1599 (2006).
Zhou, X. et al. Novel mechanisms of T-cell and dendritic cell activation revealed by profiling of psoriasis on the 63,100-element oligonucleotide array. Physiol. Genomics 13, 69–78 (2003).
Weninger, W. et al. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J. Immunol. 170, 4638–4648 (2003).
Weninger, W. & von Andrian, U. H. Chemokine regulation of naive T cell traffic in health and disease. Semin. Immunol. 15, 257–270 (2003).
McKenzie, B. S., Kastelein, R. A. & Cua, D. J. Understanding the IL-23–IL-17 immune pathway. Trends Immunol. 27, 17–23 (2006).
Nickoloff, B. J., Bonish, B., Huang, B. B. & Porcelli, S. A. Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. J. Dermatol. Sci. 24, 212–225 (2000).
Prinz, J. C. et al. T cell clones from psoriasis skin lesions can promote keratinocyte proliferation in vitro via secreted products. Eur. J. Immunol. 24, 593–598 (1994).
Sugiyama, H. et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J. Immunol. 174, 164–173 (2005).
Gottlieb, S. L. et al. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nature Med. 1, 442–447 (1995).
Abrams, J. R. et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J. Clin. Invest. 103, 1243–1252 (1999).
Abrams, J. R. et al. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells, and endothelial cells. J. Exp. Med. 192, 681–694 (2000).
Wolk, K. et al. IL-22 increases the innate immunity of tissues. Immunity 21, 241–254 (2004).
Finch, P. W., Murphy, F., Cardinale, I. & Krueger, J. G. Altered expression of keratinocyte growth factor and its receptor in psoriasis. Am. J. Pathol. 151, 1619–1628 (1997).
Bowcock, A. M. The genetics of psoriasis and autoimmunity. Annu. Rev. Genomics Hum. Genet. 6, 93–122 (2005).
Elder, J. T. et al. The genetics of psoriasis. Arch. Dermatol. 130, 216–224 (1994).
Tiilikainen, A., Lassus, A., Karvonen, J., Vartiainen, P. & Julin, M. Psoriasis and HLA-Cw6. Br. J. Dermatol. 102, 179–184 (1980).
Veal, C. D. et al. Family-based analysis using a dense single-nucleotide polymorphism-based map defines genetic variation at PSORS1, the major psoriasis-susceptibility locus. Am. J. Hum. Genet. 71, 554–564 (2002).
Nair, R. P. et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am. J. Hum. Genet. 78, 827–851 (2006).
Helms, C. et al. Localization of PSORS1 to a haplotype block harboring HLA-C and distinct from corneodesmosin and HCR. Hum. Genet. 118, 466–476 (2005).
Helms, C. et al. A putative RUNX1 binding site variant between SLC9A3R1 and NAT9 is associated with susceptibility to psoriasis. Nature Genet. 35, 349–356 (2003).
Huffmeier, U. et al. Evidence for susceptibility determinant(s) to psoriasis vulgaris in or near PTPN22 in German patients. J. Med. Genet. 43, 517–522 (2006).
Tsunemi, Y. et al. Interleukin-12 p40 gene (IL12B) 3′-untranslated region polymorphism is associated with susceptibility to atopic dermatitis and psoriasis vulgaris. J. Dermatol. Sci. 30, 161–166 (2002).
Koks, S. et al. Combined haplotype analysis of the interleukin-19 and -20 genes: relationship to plaque-type psoriasis. Genes Immun. 5, 662–667 (2004).
Foerster, J. et al. Evaluation of the IRF-2 gene as a candidate for PSORS3. J. Invest. Dermatol. 122, 61–64 (2004).
Tomfohrde, J. et al. Gene for familial psoriasis susceptibility mapped to the distal end of human chromosome 17q. Science 264, 1141–1145 (1994).
Hwu, W. L. et al. Mapping of psoriasis to 17q terminus. J. Med. Genet. 42, 152–158 (2005).
Birnbaum, R. Y. et al. Seborrhea-like dermatitis with psoriasiform elements caused by a mutation in ZNF750, encoding a putative C2H2 zinc finger protein. Nature Genet. 38, 749–751 (2006).
Nestle, F. O. & Nickoloff, B. J. From classical mouse models of psoriasis to a spontaneous xenograft model featuring use of AGR mice. Ernst Schering Res. Found. Workshop 203–212 (2005).
Villadsen, L. S. et al. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J. Clin. Invest. 112, 1571–1580 (2003).
Weinberg, J. M., Bottino, C. J., Lindholm, J. & Buchholz, R. Biologic therapy for psoriasis: an update on the tumor necrosis factor inhibitors infliximab, etanercept, and adalimumab, and the T-cell-targeted therapies efalizumab and alefacept. J. Drugs Dermatol. 4, 544–555 (2005).
Gottlieb, A. B. Psoriasis: emerging therapeutic strategies. Nature Rev. Drug Discov. 4, 19–34 (2005).
Papp, K. A. The long-term efficacy and safety of new biological therapies for psoriasis. Arch. Dermatol. Res. 298, 7–15 (2006).
Ellis, C. N. & Krueger, G. G. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N. Engl. J. Med. 345, 248–255 (2001).
Chamian, F. et al. Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris. Proc. Natl Acad. Sci. USA 102, 2075–2080 (2005).
Vugmeyster, Y. et al. Efalizumab (anti-CD11a)-induced increase in leukocyte numbers in psoriasis patients is preferentially mediated by blocked entry of memory CD8+ T cells into the skin. Clin. Immunol. 113, 38–46 (2004).
Gottlieb, A. B. et al. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J. Immunol. 175, 2721–2729 (2005).
Kruger-Krasagakis, S., Galanopoulos, V. K., Giannikaki, L., Stefanidou, M. & Tosca, A. D. Programmed cell death of keratinocytes in infliximab-treated plaque-type psoriasis. Br. J. Dermatol. 154, 460–466 (2006).
Boruchov, A. M. et al. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J. Clin. Invest. 115, 2914–2923 (2005).
Dupasquier, M., Stoitzner, P., van Oudenaren, A., Romani, N. & Leenen, P. J. Macrophages and dendritic cells constitute a major subpopulation of cells in the mouse dermis. J. Invest. Dermatol. 123, 876–879 (2004).
Chen, D. M., Gordon, K., Leonardi, C. & Menter, M. A. Adalimumab efficacy and safety in patients with moderate to severe chronic plaque psoriasis: preliminary findings from a 12-week dose-ranging trial. J. Am. Acad. Dermatol. 50, 1 (2004).
Gottlieb, A. B. et al. Oral pimecrolimus in the treatment of moderate to severe chronic plaque-type psoriasis: a double-blind, multicentre, randomized, dose-finding trial. Br. J. Dermatol. 152, 1219–1227 (2005).
Toichi, E. et al. An anti-IL-12p40 antibody down-regulates type 1 cytokines, chemokines, and IL-12/IL-23 in psoriasis. J. Immunol. 177, 4917–4926 (2006).
Xia, Y. P. et al. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood 102, 161–168 (2003).
Blumberg, H. et al. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell 104, 9–19 (2001).
Zenz, R. et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature 437, 369–375 (2005).
Haider, A. S., Duculan, J., Whynot, J. A. & Krueger, J. G. Increased JunB mRNA and protein expression in psoriasis vulgaris lesions. J. Invest. Dermatol. 126, 912–914 (2006).
Zheng, Y. et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature advance online publication (24 December 2006) doi:10.1038/nature05505.
Acknowledgements
The authors and their primary research have been supported by grants from the NIH.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
James Krueger acknowledges research support from several companies (Genentech, Serono, Amgen, Biogen, Protein Design Lab and Bristol-Myers) that market biological drugs for psoriasis.
Additional information
Note added in proof: A recent study66 shows that IL-23 induces marked hyperplasia in epidermal keratinocytes in murine skin, and results suggest that this effect is mediated to a significant extent through IL-22 produced by TH17 T cells. However, keratinocyte hyperplasia is still present on an Il22-null background, which suggests that IL-23 or other factors independent of IL-22 also stimulate keratinocyte proliferation.
Rights and permissions
About this article
Cite this article
Lowes, M., Bowcock, A. & Krueger, J. Pathogenesis and therapy of psoriasis. Nature 445, 866–873 (2007). https://doi.org/10.1038/nature05663
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05663
This article is cited by
-
miR-181a/b-5p negatively regulates keratinocytes proliferation by targeting MELK
Archives of Dermatological Research (2024)
-
Single-atom catalysts-based catalytic ROS clearance for efficient psoriasis treatment and relapse prevention via restoring ESR1
Nature Communications (2023)
-
N-eicosapentaenoyl-ethanolamine decreases the proliferation of psoriatic keratinocytes in a reconstructed psoriatic skin model
Scientific Reports (2023)
-
FXYD3 enhances IL-17A signaling to promote psoriasis by competitively binding TRAF3 in keratinocytes
Cellular & Molecular Immunology (2023)
-
SLC35E1 promotes keratinocyte proliferation in psoriasis by regulating zinc homeostasis
Cell Death & Disease (2023)