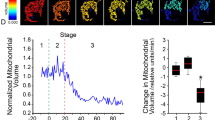
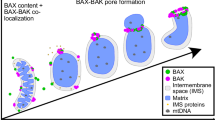
Abstract
Bcl-2 family proteins are potent regulators of programmed cell death. Although their intracellular localization to mitochondria and the endoplasmic reticulum has focused research on these organelles, how they function remains unknown. Two members of the Bcl-2 family, Bax and Bak, change intracellular location early in the promotion of apoptosis to concentrate in focal clusters at sites of mitochondrial division. Here we report that in healthy cells Bax or Bak is required for normal fusion of mitochondria into elongated tubules. Bax seems to induce mitochondrial fusion by activating assembly of the large GTPase Mfn2 and changing its submitochondrial distribution and membrane mobility—properties that correlate with different GTP-bound states of Mfn2. Our results show that Bax and Bak regulate mitochondrial dynamics in healthy cells and indicate that Bcl-2 family members may also regulate apoptosis through organelle morphogenesis machineries.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cory, S., Huang, D. C. & Adams, J. M. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22, 8590–8607 (2003)
Martinou, J. C. & Green, D. R. Breaking the mitochondrial barrier. Nature Rev. Mol. Cell Biol. 2, 63–67 (2001)
Wei, M. C. et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 (2001)
Karbowski, M. et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 159, 931–938 (2002)
Degenhardt, K., Sundararajan, R., Lindsten, T., Thompson, C. & White, E. Bax and Bak independently promote cytochrome c release from mitochondria. J. Biol. Chem. 277, 14127–14134 (2002)
Chen, H. et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 160, 189–200 (2003)
Lum, J. J. et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120, 237–248 (2005)
Szabadkai, G. et al. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol. Cell 16, 59–68 (2004)
Arnoult, D. et al. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl Acad. Sci. USA 101, 7988–7993 (2004)
Poncet, D. et al. An anti-apoptotic viral protein that recruits Bax to mitochondria. J. Biol. Chem. 279, 22605–22614 (2004)
McCormick, A. L., Smith, V. L., Chow, D. & Mocarski, E. S. Disruption of mitochondrial networks by the human cytomegalovirus UL37 gene product viral mitochondrion-localized inhibitor of apoptosis. J. Virol. 77, 631–641 (2003)
van der Bliek, A. M. A mitochondrial division apparatus takes shape. J. Cell Biol. 151, F1–F4 (2000)
Shaw, J. M. & Nunnari, J. Mitochondrial dynamics and division in budding yeast. Trends Cell Biol. 12, 178–184 (2002)
Karbowski, M., Jeong, S. Y. & Youle, R. J. Endophilin B1 is required for the maintenance of mitochondrial morphology. J. Cell Biol. 166, 1027–1039 (2004)
Neuspiel, M., Zunino, R., Gangaraju, S., Rippstein, P. & McBride, H. M. Activated Mfn2 signals mitochondrial fusion, interferes with Bax activation and reduces susceptibility to radical induced depolarization. J. Biol. Chem. 280, 25060–25070 (2005)
Koshiba, T. et al. Structural basis of mitochondrial tethering by mitofusin complexes. Science 305, 858–862 (2004)
Karbowski, M. et al. Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J. Cell Biol. 164, 493–499 (2004)
Majewski, N. et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol. Cell 16, 819–830 (2004)
Ishihara, N., Eura, Y. & Mihara, K. Mitofusion 1 and 2 play distinct roles in mitochondria fusion reactions via GTPase activity. J. Cell Sci. 117, 6535–6546 (2004)
Takahashi, Y. et al. Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis. Mol. Cell. Biol. 25, 9369–9382 (2005)
Youle, R. J. & Karbowski, M. Mitochondrial fission in apoptosis. Nature Rev. Mol. Cell Biol. 6, 657–663 (2005)
Sugioka, R., Shimizu, S. & Tsujimoto, Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J. Biol. Chem. 279, 52726–52734 (2004)
Jagasia, R., Grote, P., Westermann, B. & Conradt, B. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature 433, 754–760 (2005)
Delivani, P., Adrain, C., Taylor, R. C., Duriez, P. J. & Martin, S. J. Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol. Cell 21, 761–773 (2006)
Acknowledgements
We thank N. Swarup for help in the early stages of this work; C. Thompson, J. Lum and T. Lindsten for Bax/Bak DKO bone marrow cells, Bak-transfected DKO cells and DKO primary MEFs; E. White, S. Korsmeyer and D. Chan for Bax/Bak DKO BMK cells, Bax/Bak DKO MEFs and Mfn KO MEFs, respectively; B. Vogelstein for HCT116 Bax-/- cells, J. Norris for DU145 cells, V. Goldmacher for vMIA HeLa cells, H. McBride for antibodies to Mfn2 and mutant Mfn2 constructs; P. Hajek and G. Attardi for antibodies to Mfn2; A. Antignani, A. Neutzner, B. Fell, S.-W. Ryu and S. Smith for technical assistance; and C. Smith for assistance with confocal microscopy and data analysis and for reading the manuscript. This work was supported by the Intramural Research Program of the NIH, National Institute of Neurological Disorders and Stroke. Author Contributions M.K., K.N. and R.Y. contributed to the conception, interpretation, execution and presentation of the experiments; S-Y.J. contributed to the RNAi experiments; M.C. participated in FRAP experiments and data analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains Supplementary Methods and Supplementary Figure Legends (DOC 107 kb)
Supplementary Figure 1
Quantification of mitochondrial morphology with and without Bax/Bak expression in baby mouse kidney cells (BMK) and primary mouse embryonic fibroblasts (MEFs). (JPG 16 kb)
Supplementary Figure 2
Development and mitochondrial phenotype of Bax and Bak double RNAi cells (JPG 22 kb)
Supplementary Figure 3
Viral mitochondria-associated inhibitor of apoptosis (vMIA) interacts with both Bax and Bak and inhibits apoptosis induced by Bak-overexpression (JPG 17 kb)
Supplementary Figure 4
Certain Bcl-2 family members other than Bax do not reverse vMIA-induced mitochondrial fragmentation (JPG 14 kb)
Supplementary Figure 5
Blocking fission does not reverse fragmentation induced by vMIA expression or loss of Bax/Bak (JPG 63 kb)
Supplementary Figure 6
vMIA blocks mitochondrial fusion (JPG 26 kb)
Supplementary Figure 7
Drp1 RNAi effect on mitochondria in Bax/Bak DKO MEFs (JPG 12 kb)
Supplementary Figure 8
Expression and sub-cellular distribution of mitochondrial fusion and fission proteins in 129/CD1 WT and 129/CD1 Bax/Bak DKO MEFs (JPG 10 kb)
Supplementary Figure 9
Quantification of Mfn2-YFP sub-mitochondrial localization in WT and Bax/Bak DKO MEFs (JPG 10 kb)
Supplementary Figure 10
FRAP of mitochondrial outer membrane-associated proteins in Bax/Bak DKO MEFs (JPG 17 kb)
Supplementary Figure 11a-d
Sub-mitochondrial distribution of YFP chimeras of several Mfn2 mutants. (JPG 27 kb)
Supplementary Figure 11e-h
Sub-mitochondrial distribution of YFP chimeras of several Mfn2 mutants. (JPG 54 kb)
Supplementary Figure 12
Opa1 complex formation is not altered in WT and Bax/Bak DKO MEFs (JPG 9 kb)
Supplementary Figure 13
Gel Filtration analysis of Mfn2 complex formation (JPG 10 kb)
Supplementary Figure 14
Mitochondrial membrane potential (Δψm) and ATP levels in WT and Bax/Bak DKO MEFs (JPG 15 kb)
Rights and permissions
About this article
Cite this article
Karbowski, M., Norris, K., Cleland, M. et al. Role of Bax and Bak in mitochondrial morphogenesis. Nature 443, 658–662 (2006). https://doi.org/10.1038/nature05111
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05111
This article is cited by
-
Cell-specific modulation of mitochondrial respiration and metabolism by the pro-apoptotic Bcl-2 family members Bax and Bak
Apoptosis (2024)
-
Targeting multifunctional magnetic nanowires for drug delivery in cancer cell death: an emerging paradigm
Environmental Science and Pollution Research (2023)
-
Sex Hormone-Binding Globulin (SHBG) Maintains Proper Equine Adipose-Derived Stromal Cells (ASCs)’ Metabolic Functions and Negatively Regulates their Basal Adipogenic Potential
Stem Cell Reviews and Reports (2023)
-
Necroptosis, pyroptosis and apoptosis: an intricate game of cell death
Cellular & Molecular Immunology (2021)
-
Exploring the multiple roles of guardian of the genome: P53
Egyptian Journal of Medical Human Genetics (2020)