Abstract
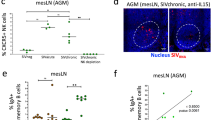
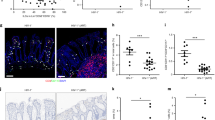
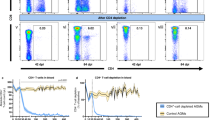
In early simian immunodeficiency virus (SIV) and human immunodeficiency virus-1 (HIV-1) infections, gut-associated lymphatic tissue (GALT), the largest component of the lymphoid organ system1, is a principal site of both virus production and depletion of primarily lamina propria memory CD4+ T cells; that is, CD4-expressing T cells that previously encountered antigens and microbes and homed to the lamina propria of GALT2,3,4,5,6,7,8,9. Here, we show that peak virus production in gut tissues of SIV-infected rhesus macaques coincides with peak numbers of infected memory CD4+ T cells. Surprisingly, most of the initially infected memory cells were not, as expected10,11, activated but were instead immunophenotypically ‘resting’ cells that, unlike truly resting cells, but like the first cells mainly infected at other mucosal sites and peripheral lymph nodes12,13, are capable of supporting virus production. In addition to inducing immune activation and thereby providing activated CD4+ T-cell targets to sustain infection, virus production also triggered14 an immunopathologically limiting Fas–Fas-ligand-mediated apoptotic pathway15,16 in lamina propria CD4+ T cells, resulting in their preferential ablation. Thus, SIV exploits a large, resident population of resting memory CD4+ T cells in GALT to produce peak levels of virus that directly (through lytic infection) and indirectly (through apoptosis of infected and uninfected cells) deplete CD4+ T cells in the effector arm of GALT. The scale of this CD4+ T-cell depletion has adverse effects on the immune system of the host, underscoring the importance of developing countermeasures to SIV that are effective before infection of GALT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mowat, A. M. & Viney, J. L. The anatomical basis of intestinal immunity. Immunol. Rev. 156, 145–166 (1997)
Veazey, R. S. et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280, 427–431 (1998)
Kewenig, S. et al. Rapid CD4+ T cell depletion and enteropathy in simian immunodeficiency virus infected rhesus macaques. Gastroenterology 116, 1115–1123 (1999)
Schneider, T. et al. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Gut 37, 524–529 (1995)
Mehandru, S. et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200, 761–770 (2004)
Brenchley, J. M. et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200, 749–759 (2004)
Guadalupe, M. et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77, 11708–11717 (2003)
Clayton, F., Snow, G., Reka, S. & Kotler, D. P. Selective depletion of rectal lamina propria rather than lymphoid aggregate CD4 lymphocytes in HIV infection. Clin. Exp. Immunol. 107, 288–292 (1997)
Lim, S. G. et al. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin. Exp. Immunol. 92, 448–454 (1993)
Veazey, R. & Lackner, A. The mucosal immune system and HIV-1 infection. AIDS Rev. 5, 245–252 (2003)
Veazey, R. et al. Identifying the target cell in primary simian immunodeficiency virus infection: Highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo . J. Virol. 74, 57–64 (2000)
Zhang, Z.-Q. et al. Sexual transmission and propagation of simian and human immunodeficiency viruses in two distinguishable populations of CD4+ T cells. Science 286, 1353–1357 (1999)
Zhang, Z.-Q. et al. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc. Natl Acad. Sci. USA 101, 5640–5645 (2004)
Boirivant, M. et al. HIV-1 gp120 accelerates Fas-mediated activation-induced human lamina propria T cell apoptosis. J. Clin. Immunol. 18, 39–47 (1998)
Boirivant, M. et al. Stimulated human lamina propria T cells manifest enhanced Fas-mediated apoptosis. J. Clin. Invest. 98, 2616–2622 (1996)
De Maria, R. et al. Functional expression of Fas and Fas ligand on human gut lamina propria T lymphocytes. A potential role for the acidic sphingomyelinase pathway in normal immunoregulation. J. Clin. Invest. 97, 316–322 (1996)
Testi, R., Phillips, J. H. & Lanier, L. L. Constitutive expression of a phosphorylated activation antigen (Leu 23) by CD3 bright human thymocytes. J. Immunol. 141, 2557–2563 (1988)
Testi, R., Phillips, J. H. & Lanier, L. L. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular [Ca + + ] and stimulation of protein kinase C. J. Immunol. 142, 1854–1860 (1989)
Phillips, A. N. Reduction of HIV concentration during acute infection: independence from a specific immune response. Science 271, 497–499 (1996)
Reilly, C. S. et al. The clustering of SIV infected cells in lymphatic tissue. J. Am. Stat. Assoc. 97, 943–954 (2002)
Mothe, B. R. et al. Dominance of CD8 responses specific for epitopes bound by a single major histocompatibility complex class I molecule during the acute phase of viral infection. J. Virol. 76, 875–884 (2002)
Reynolds, M. R. et al. The CD8+ lymphocyte response to major immunodominant epitopes after vaginal exposure to SIV: too late and too little. J. Virol (in the press)
Pope, M. & Haase, A. T. Transmission, acute HIV-1 infection and the quest for effective vaccines, microbicides and other strategies to prevent infection. Nature Med. 9, 847–852 (2003)
Miller, C. J. et al. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J. Virol. 68, 6391–6400 (1994)
Poppema, S., Lai, R. & Visser, L. Monoclonal antibody OPD4 is reactive with CD45RO, but differs from UCHL1 by the absence of monocyte reactivity. Am. J. Pathol. 139, 725–729 (1991)
Miller, C. J. et al. Propagation and dissemination of infection after vaginal transmission of SIV. J. Virol (submitted)
Acknowledgements
We thank R. Veazey, L. Picker, J. Lifson, D. Douek and M. Roederer for discussions; L. Compton, D. Lu, B. Vang, K. Bost and R. Dizon of the Immunology Core Laboratory and Primate Services Unit at the CNPRC for technical assistance; and T. Leonard and C. O'Neill for help in preparing the figures and manuscript. This work was supported by grants from the National Institute of Allergy and Infectious Diseases and from the National Center for Research Resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Li, Q., Duan, L., Estes, J. et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434, 1148–1152 (2005). https://doi.org/10.1038/nature03513
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature03513