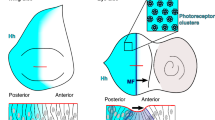
Abstract
In many developmental contexts, a locally produced morphogen specifies positional information by forming a concentration gradient over a field of cells1. However, during embryonic dorsal–ventral patterning in Drosophila, two members of the bone morphogenetic protein (BMP) family, Decapentaplegic (Dpp) and Screw (Scw), are broadly transcribed but promote receptor-mediated signalling in a restricted subset of expressing cells2,3,4. Here we use a novel immunostaining protocol to visualize receptor-bound BMPs and show that both proteins become localized to a sharp stripe of dorsal cells. We demonstrate that proper BMP localization involves two distinct processes. First, Dpp undergoes directed, long-range extracellular transport. Scw also undergoes long-range movement, but can do so independently of Dpp transport. Second, an intracellular positive feedback circuit promotes future ligand binding as a function of previous signalling strength. These data elicit a model in which extracellular Dpp transport initially creates a shallow gradient of BMP binding that is acted on by positive intracellular feedback to produce two stable states of BMP–receptor interactions, a spatial bistability in which BMP binding and signalling capabilities are high in dorsal-most cells and low in lateral cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tabata, T. & Takei, Y. Morphogens, their identification and regulation. Development 131, 703–712 (2004)
St Johnston, R. D. & Gelbart, W. M. Decapentaplegic transcripts are localized along the dorsal-ventral axis of the Drosophila embryo. EMBO J. 6, 2785–2791 (1987)
Arora, K., Levine, M. S. & O'Connor, M. B. The screw gene encodes a ubiquitously expressed member of the TGF-β family required for specification of dorsal cell fates in the Drosophila embryo. Genes Dev. 8, 2588–2601 (1994)
Dorfman, R. & Shilo, B. Z. Biphasic activation of the BMP pathway patterns the Drosophila embryonic dorsal region. Development 128, 965–972 (2001)
Irish, V. F. & Gelbart, W. M. The decapentaplegic gene is required for dorsal-ventral patterning of the Drosophila embryo. Genes Dev. 1, 868–879 (1987)
Rushlow, C., Colosimo, P. F., Lin, M. C., Xu, M. & Kirov, N. Transcriptional regulation of the Drosophila gene zen by competing Smad and Brinker inputs. Genes Dev. 15, 340–351 (2001)
Ross, J. J. et al. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature 410, 479–483 (2001)
Ferguson, E. L. & Anderson, K. V. Decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell 71, 451–461 (1992)
Brummel, T. J. et al. Characterization and relationship of Dpp receptors encoded by the saxophone and thick veins genes in Drosophila. Cell 78, 251–261 (1994)
Penton, A. et al. Identification of two bone morphogenetic protein type I receptors in Drosophila and evidence that Brk25D is a decapentaplegic receptor. Cell 78, 239–250 (1994)
Letsou, A. et al. Drosophila Dpp signaling is mediated by the punt gene product: a dual ligand-binding type II receptor of the TGF-β receptor family. Cell 80, 899–908 (1995)
Teleman, A. A. & Cohen, S. M. Dpp gradient formation in the Drosophila wing imaginal disc. Cell 103, 971–980 (2000)
Kosman, D. & Small, S. Concentration-dependent patterning by an ectopic expression domain of the Drosophila gap gene knirps. Development 124, 1343–1354 (1997)
Decotto, E. & Ferguson, E. L. A positive role for Short gastrulation in modulating BMP signaling during dorsoventral patterning in the Drosophila embryo. Development 128, 3831–3841 (2001)
Eldar, A. et al. Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature 419, 304–308 (2002)
Shimmi, O. & O'Connor, M. B. Physical properties of Tld, Sog, Tsg and Dpp protein interactions are predicted to help create a sharp boundary in Bmp signals during dorsoventral patterning of the Drosophila embryo. Development 130, 4673–4682 (2003)
Holley, S. A. et al. The Xenopus dorsalizing factor Noggin ventralizes Drosophila embryos by preventing DPP from activating its receptor. Cell 86, 607–617 (1996)
Ashe, H. L. & Levine, M. Local inhibition and long-range enhancement of Dpp signal transduction by Sog. Nature 398, 427–431 (1999)
Marques, G. et al. Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell 91, 417–426 (1997)
Oelgeschlager, M. et al. The pro-BMP activity of Twisted gastrulation is independent of BMP binding. Development 130, 4047–4056 (2003)
Neul, J. L. & Ferguson, E. L. Spatially restricted activation of the SAX receptor by SCW modulates DPP/TKV signaling in Drosophila dorsal-ventral patterning. Cell 95, 483–494 (1998)
Nguyen, M., Park, S., Marques, G. & Arora, K. Interpretation of a BMP activity gradient in Drosophila embryos depends on synergistic signaling by two type I receptors, SAX and TKV. Cell 95, 495–506 (1998)
Shimmi, O., Umulis, D., Othmer, H. & O'Connor, M. B. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell (in the press)
Mason, E. D., Williams, S., Grotendorst, G. R. & Marsh, J. L. Combinatorial signaling by Twisted Gastrulation and Decapentaplegic. Mech. Dev. 64, 61–75 (1997)
Wisotzkey, R. G. et al. Medea is a Drosophila Smad4 homolog that is differentially required to potentiate DPP responses. Development 125, 1433–1445 (1998)
Rushlow, C., Doyle, H., Hoey, T. & Levine, M. Molecular characterization of the zerknullt region of the Antennapedia gene complex in Drosophila. Genes Dev. 1, 1268–1279 (1987)
Ferrell, J. E. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14, 140–148 (2002)
Lai, K., Robertson, M. J. & Schaffer, D. V. The Sonic hedgehog signaling system as a bistable genetic switch. Biophys. J. 86, 2748–2757 (2004)
Stein, D., Roth, S., Vogelsang, E. & Nusslein-Volhard, C. The polarity of the dorsoventral axis in the Drosophila embryo is defined by an extracellular signal. Cell 65, 725–735 (1991)
Little, S. C. & Mullins, M. C. Twisted gastrulation promotes BMP signaling in zebrafish dorsal-ventral axial patterning. Development 131, 5825–5835 (2004)
Xie, J. & Fisher, S. Twisted gastrulation enhances BMP signaling through chordin dependent and independent mechanisms. Development 132, 383–391 (2005)
Acknowledgements
We thank N. Dostatni, M. Levine and M. Markstein for fly stocks; S. Cohen, M. Levine and M. Markstein for plasmids; P. ten Dijke and C. Heldin for antibodies; V. Bindokas for assistance with confocal microscopy; S. Lemke and M. Markstein for discussions; D. Bishop, M. O. Casanueva, C. Li, A. Mahowald, J. Malamy, M. Markstein, V. Prince and J. Staley for helpful comments on the manuscript, and M. O'Connor for sharing unpublished data and reagents. Preliminary data on the second function of Tsg were obtained by E. Decotto. Funding was provided by grants to E.L.F. from the NIH and from the Human Frontiers Science program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure S1
This figure shows that there is a limited amount of Dpp diffusion in the absence of Sog. (DOC 1126 kb)
Supplementary Figure S2
This figure shows that Tsg promotes BMP signalling independent of Sog (DOC 1459 kb)
Supplementary Figure S3
This figure presents a characterization of sog; scw mutant embryos. (DOC 771 kb)
Supplementary Figure S4
This figure shows the relationship between tld and sog expression patterns and BMP signaling. (DOC 1209 kb)
Supplementary Figure S5
This figure shows that the pattern of tld expression is not affected before the onset of gastrulation in a dpp null embryo. (DOC 668 kb)
Supplementary Methods
This document contains additional details of the crosses that were performed to generate certain embryonic genotypes and additional information concerning the concentration of different primary and secondary antibodies used in these experiments. (DOC 50 kb)
Rights and permissions
About this article
Cite this article
Wang, YC., Ferguson, E. Spatial bistability of Dpp–receptor interactions during Drosophila dorsal–ventral patterning. Nature 434, 229–234 (2005). https://doi.org/10.1038/nature03318
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03318
This article is cited by
-
Highly conserved and extremely evolvable: BMP signalling in secondary axis patterning of Cnidaria and Bilateria
Development Genes and Evolution (2024)
-
A deeper understanding of system interactions can explain contradictory field results on pesticide impact on honey bees
Nature Communications (2022)
-
Experimental duplication of bilaterian body axes in spider embryos: Holm’s organizer and self-regulation of embryonic fields
Development Genes and Evolution (2020)
-
The Role of Mathematical Models in Understanding Pattern Formation in Developmental Biology
Bulletin of Mathematical Biology (2015)
-
Ancient and diverged TGF-β signaling components in Nasonia vitripennis
Development Genes and Evolution (2014)