Abstract
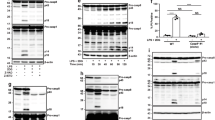
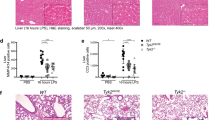
Specific adaptors regulate the activation of initiator caspases; for example, FADD and Apaf-1 engage caspases 8 and 9, respectively1. The adaptors ASC, Ipaf and RIP2 have each been proposed to regulate caspase-1 (also called interleukin (IL)-1 converting enzyme), which is activated within the ‘inflammasome’, a complex comprising several adaptors2. Here we show the impact of ASC-, Ipaf- or RIP2-deficiency on inflammasome function. ASC was essential for extracellular ATP-driven activation of caspase-1 in toll-like receptor (TLR)-stimulated macrophages. Accordingly, ASC-deficient macrophages exhibited defective maturation of IL-1β and IL-18, and ASC-null mice were resistant to lipopolysaccharide-induced endotoxic shock. Furthermore, activation of caspase-1 in response to an intracellular pathogen (Salmonella typhimurium) was abrogated severely in ASC-null macrophages. Unexpectedly, Ipaf-deficient macrophages activated caspase-1 in response to TLR plus ATP stimulation but not S. typhimurium. Caspase-1 activation was not compromised by loss of RIP2. These data show that whereas ASC is key to caspase-1 activation within the inflammasome, Ipaf provides a special conduit to the inflammasome for signals triggered by intracellular pathogens. Notably, cell death triggered by stimuli that engage caspase-1 was ablated in macrophages lacking either ASC or Ipaf, suggesting a coupling between the inflammatory and cell death pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ashkenazi, A. & Dixit, V. M. Death receptors: signaling and modulation. Science 281, 1305–1308 (1998)
Tschopp, J., Martinon, F. & Burns, K. NALPs: a novel protein family involved in inflammation. Nature Rev. Mol. Cell Biol. 4, 95–104 (2003)
Dinarello, C. A. Interleukin-1β, interleukin-18, and the interleukin-1β converting enzyme. Ann. NY Acad. Sci. 856, 1–11 (1998)
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002)
Masumoto, J. et al. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J. Biol. Chem. 274, 33835–33838 (1999)
Conway, K. E. et al. TMS1, a novel proapoptotic caspase recruitment domain protein, is a target of methylation-induced gene silencing in human breast cancers. Cancer Res. 60, 6236–6242 (2000)
Srinivasula, S. M. et al. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J. Biol. Chem. 19, 19–22 (2002)
Wang, L. et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-κβ and caspase-1-dependent cytokine processing. J. Biol. Chem. 277, 29874–29880 (2002)
Stehlik, C. et al. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J. Immunol. 171, 6154–6163 (2003)
Hogquist, K. A., Nett, M. A., Unanue, E. R. & Chaplin, D. D. Interleukin 1 is processed and released during apoptosis. Proc. Natl Acad. Sci. USA 88, 8485–8489 (1991)
Perregaux, D. & Gabel, C. A. Interleukin-1β maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 269, 15195–15203 (1994)
Chen, Y., Smith, M. R., Thirumalai, K. & Zychlinsky, A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 15, 3853–3860 (1996)
Hersh, D. et al. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl Acad. Sci. USA 96, 2396–2401 (1999)
Thornberry, N. A. et al. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature 356, 768–774 (1992)
Singer, I. I. et al. The interleukin-1β-converting enzyme (ICE) is localized on the external cell surface membranes and in the cytoplasmic ground substance of human monocytes by immuno-electron microscopy. J. Exp. Med. 182, 1447–1459 (1995)
MacKenzie, A. et al. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity 15, 825–835 (2001)
Li, P. et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80, 401–411 (1995)
Kuida, K. et al. Altered cytokine export and apoptosis in mice deficient in interleukin- 1β converting enzyme. Science 267, 2000–2003 (1995)
Stehlik, C. et al. The PAAD/PYRIN-family protein ASC is a dual regulator of a conserved step in nuclear factor κB activation pathways. J. Exp. Med. 196, 1605–1615 (2002)
Humke, E. W., Shriver, S. K., Starovasnik, M. A., Fairbrother, W. J. & Dixit, V. M. ICEBERG: a novel inhibitor of interleukin-1β generation. Cell 103, 99–111 (2000)
Thome, M. et al. Identification of CARDIAK, a RIP-like kinase that associates with caspase-1. Curr. Biol. 8, 885–888 (1998)
Geddes, B. J. et al. Human CARD12 is a novel CED4/Apaf-1 family member that induces apoptosis. Biochem. Biophys. Res. Commun. 284, 77–82 (2001)
Poyet, J. L. et al. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J. Biol. Chem. 276, 28309–28313 (2001)
Kobayashi, K. et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416, 194–199 (2002)
Damiano, J. S., Stehlik, C., Pio, F., Godzik, A. & Reed, J. C. CLAN, a novel human CED-4-like gene. Genomics 75, 77–83 (2001)
Monack, D. M. et al. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J. Exp. Med. 192, 249–258 (2000)
Jesenberger, V., Procyk, K. J., Yuan, J., Reipert, S. & Baccarini, M. Salmonella-induced caspase-2 activation in macrophages: a novel mechanism in pathogen-mediated apoptosis. J. Exp. Med. 192, 1035–1046 (2000)
Gumucio, D. L. et al. Fire and ICE: the role of pyrin domain-containing proteins in inflammation and apoptosis. Clin. Exp. Rheumatol. 20, S45–S53 (2002)
Inohara, N. & Nunez, G. NODs: intracellular proteins involved in inflammation and apoptosis. Nature Rev. Immunol. 3, 371–382 (2003)
Ruefli-Brasse, A. A., Lee, W. P., Hurst, S. & Dixit, V. M. Rip2 participates in Bcl10 signaling and T-cell receptor-mediated NF-κB activation. J. Biol. Chem. 279, 1570–1574 (2004)
Acknowledgements
We thank D. Dornan and other members of the Dixit laboratory for discussions, E. Humke for help with illustrations, and K. O'Rourke, D. Wadley, Z. Gu, C. Olsson, M. Bauer, L. Tom, J. Kloss, M. Fuentes, M. Osborn, C. Tan, J. Hongo, T. Wong and A. Chuntharapai for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
Targeted disruption of the murine asc gene. (PDF 397 kb)
Supplementary Figure 2
LPS stimulation is not necessary for caspase-1 activation by S. typhimurium. (PDF 336 kb)
Supplementary Figure 3
Association of ASC and caspase-1 was not detected in macrophage cell lysates. (PDF 767 kb)
Supplementary Figure 4
Both TLR stimulation and extracellular ATP are required for caspase-1 activation and IL-1β release by macrophages. (PDF 870 kb)
Supplementary Figure 5
Defective secretion of IL-1β by asc-/- macrophages is due to defective maturation of pro-IL-1β rather than impaired release of mature IL-1β. (PDF 684 kb)
Supplementary Figure 6
ASC-deficient mice challenged with LPS and ATP produce less IL-α, IL-1β and IL-18 than their wild-type counterparts. (PDF 214 kb)
Supplementary Figure 7
ASC is dispensable for normal LPS- and TNF-induced IKK and ERK activation. (PDF 775 kb)
Supplementary Figure 8
RIP2 is dispensable for caspase-1 activation. (PDF 503 kb)
Supplementary Figure 9
Generation of Ipaf-deficient mice. (PDF 279 kb)
Supplementary Figure 10
Expression of ASC and Ipaf is not effected in Ipaf-null macrophages and ASC-null macrophages, respectively. (PDF 943 kb)
Rights and permissions
About this article
Cite this article
Mariathasan, S., Newton, K., Monack, D. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004). https://doi.org/10.1038/nature02664
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature02664
This article is cited by
-
The role of inflammasomes in human diseases and their potential as therapeutic targets
Signal Transduction and Targeted Therapy (2024)
-
A cytomegalovirus inflammasome inhibitor reduces proinflammatory cytokine release and pyroptosis
Nature Communications (2024)
-
Involvement of inflammasomes in tumor microenvironment and tumor therapies
Journal of Hematology & Oncology (2023)
-
Inflammasomes as regulators of mechano-immunity
EMBO Reports (2023)
-
ASC- and caspase-1-deficient C57BL/6 mice do not develop demyelinating disease after infection with Theiler’s murine encephalomyelitis virus
Scientific Reports (2023)