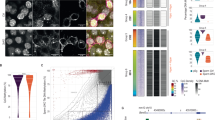
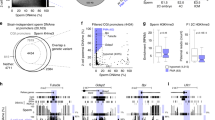
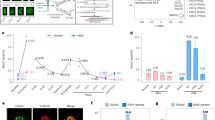
Abstract
Imprinted genes are epigenetically marked during gametogenesis so that they are exclusively expressed from either the paternal or the maternal allele in offspring1. Imprinting prevents parthenogenesis in mammals and is often disrupted in congenital malformation syndromes, tumours and cloned animals1. Although de novo DNA methyltransferases of the Dnmt3 family are implicated in maternal imprinting2, the lethality of Dnmt3a and Dnmt3b knockout mice3 has precluded further studies. We here report the disruption of Dnmt3a and Dnmt3b in germ cells, with their preservation in somatic cells, by conditional knockout technology4. Offspring from Dnmt3a conditional mutant females die in utero and lack methylation and allele-specific expression at all maternally imprinted loci examined. Dnmt3a conditional mutant males show impaired spermatogenesis and lack methylation at two of three paternally imprinted loci examined in spermatogonia. By contrast, Dnmt3b conditional mutants and their offspring show no apparent phenotype. The phenotype of Dnmt3a conditional mutants is indistinguishable from that of Dnmt3L knockout mice2,5, except for the discrepancy in methylation at one locus. These results indicate that both Dnmt3a and Dnmt3L are required for methylation of most imprinted loci in germ cells, but also suggest the involvement of other factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Reik, W. & Walter, J. Genomic imprinting: parental influence on the genome. Nature Rev. Genet. 2, 21–32 (2001)
Hata, K., Okano, M., Lei, H. & Li, E. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development 129, 1983–1993 (2002)
Okano, M., Bell, D. W., Haber, D. A. & Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99, 247–257 (1999)
Torres, R. M. & Kühn, R. Laboratory Protocols for Conditional Gene Targeting (Oxford Univ. Press, 1997)
Bourc'his, D., Xu, G. L., Lin, C. S., Bollman, B. & Bestor, T. H. Dnmt3L and the establishment of maternal genomic imprints. Science 294, 2536–2539 (2001)
Lomeli, H., Ramos-Mejia, V., Gertsenstein, M., Lobe, C. G. & Nagy, A. Targeted insertion of Cre recombinase into the TNAP gene: excision in primordial germ cells. Genesis 26, 116–117 (2000)
Ueda, T. et al. The paternal methylation imprint of the mouse H19 locus is acquired in the gonocyte stage during foetal testis development. Genes Cells 5, 649–659 (2000)
Davis, T. L., Yang, G. J., McCarrey, J. R. & Bartolomei, M. S. The H19 methylation imprint is erased and re-established differentially on the parental alleles during male germ cell development. Hum. Mol. Genet. 9, 2885–2894 (2000)
Obata, Y. & Kono, T. Maternal primary imprinting is established at a specific time for each gene throughout oocyte growth. J. Biol. Chem. 277, 5285–5289 (2002)
Lucifero, D., Mann, M. R. W., Bartolomei, M. S. & Trasler, J. M. Gene-specific timing and epigenetic memory in oocyte imprinting. Hum. Mol. Genet. 13, 839–849 (2004)
Ferguson-Smith, A. C. & Surani, M. A. Imprinting and the epigenetic asymmetry between parental genomes. Science 293, 1086–1089 (2001)
Clark, S. J., Harrison, J., Paul, C. L. & Frommer, M. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 22, 2990–2997 (1994)
Chen, T., Ueda, Y., Xie, S. & Li, E. A novel Dnmt3a isoform produced from an alternative promoter localizes to euchromatin and its expression correlates with active de novo methylation. J. Biol. Chem. 277, 38746–38754 (2002)
Chedin, F., Lieber, M. R. & Hsieh, C. L. The DNA methyltransferase-like protein DNMT3L stimulates de novo methylation by Dnmt3a. Proc. Natl Acad. Sci. USA 99, 16916–16921 (2002)
Cerrato, F. et al. Paternal imprints can be established on the maternal Igf2–H19 locus without altering replication timing of DNA. Hum. Mol. Genet. 12, 3123–3132 (2003)
Fisher, R. A. et al. The maternally transcribed gene p57KIP2 (CDNK1C) is abnormally expressed in both androgenetic and biparental complete hydatidiform moles. Hum. Mol. Genet. 11, 3267–3272 (2002)
Judson, H., Hayward, B. E., Sheridan, E. & Bonthron, D. T. A global disorder of imprinting in the human female germ line. Nature 416, 539–542 (2002)
El-Maarri, O. et al. Maternal alleles acquiring paternal methylation patterns in biparental complete hydatidiform moles. Hum. Mol. Genet. 12, 1405–1413 (2003)
Hayward, B. E. et al. Lack of involvement of known DNA methyltransferases in familial hydatidiform mole implies the involvement of other factors in establishment of imprinting in the human female germline. BMC Genet. 4, 2 (2003)
Sado, T., Okano, M., Li, E. & Sasaki, H. De novo DNA methylation is dispensable for the initiation and propagation of X chromosome inactivation. Development 131, 975–982 (2004)
Li, E., Bestor, T. H. & Jaenisch, R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69, 915–926 (1992)
Xu, X. et al. Direct removal in the mouse of a floxed neo gene from a three-loxP conditional knockout allele by two novel approaches. Genesis 30, 1–6 (2001)
Hashimoto, N., Kubokawa, R., Yamazaki, K., Noguchi, M. & Kato, Y. Germ cell deficiency causes testis cord differentiation in reconstituted mouse fetal ovaries. J. Exp. Zool. 253, 61–70 (1990)
Bao, S., Obata, Y., Carroll, J., Domeki, I. & Kono, T. Epigenetic modifications necessary for normal development are established during oocyte growth in mice. Biol. Reprod. 62, 616–621 (2000)
Acknowledgements
We thank A. Nagy and H. Lomeli (Samuel Lunenfeld Research Institute) for providing the TNAP–Cre mice; K. Shiota and S. Tanaka (The University of Tokyo) for help in laser-microdissection microscopy; K. Kumaki and Y. Kato for help and advice on bisulphite sequencing; and C. Suda, M. Kanbayashi, M. Serizawa and H. Furuumi for technical assistance and mouse maintenance. This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to H.S., and grants from the National Institutes of Health to E.L.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure
Includes bisulfite sequencing profiles of IAP LTR sequences of oocytes and spermatogonia lacking Dnmt3a. (PDF 38 kb)
Rights and permissions
About this article
Cite this article
Kaneda, M., Okano, M., Hata, K. et al. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 429, 900–903 (2004). https://doi.org/10.1038/nature02633
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02633