Abstract
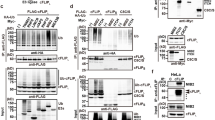
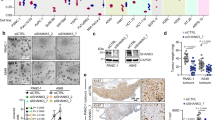
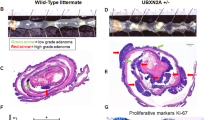
Protein modification by the conjugation of ubiquitin moieties—ubiquitination—plays a major part in many biological processes, including cell cycle and apoptosis1. The enzymes that mediate ubiquitin-conjugation have been well-studied, but much less is known about the ubiquitin-specific proteases that mediate de-ubiquitination of cellular substrates2,3. To study this gene family, we designed a collection of RNA interference vectors to suppress 50 human de-ubiquitinating enzymes, and used these vectors to identify de-ubiquitinating enzymes in cancer-relevant pathways. We report here that inhibition of one of these enzymes, the familial cylindromatosis tumour suppressor gene (CYLD)4, having no known function, enhances activation of the transcription factor NF-κB. We show that CYLD binds to the NEMO (also known as IKKγ) component of the IκB kinase (IKK) complex, and appears to regulate its activity through de-ubiquitination of TRAF2, as TRAF2 ubiquitination can be modulated by CYLD. Inhibition of CYLD increases resistance to apoptosis, suggesting a mechanism through which loss of CYLD contributes to oncogenesis. We show that this effect can be relieved by aspirin derivatives that inhibit NF-κB activity5, which suggests a therapeutic intervention strategy to restore growth control in patients suffering from familial cylindromatosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wilkinson, K. D. Ubiquitination and deubiquitination: Targeting of proteins for degradation by the proteasome. Semin. Cell Dev. Biol. 11, 141–148 (2000)
Chung, C. H. & Baek, S. H. Deubiquitinating enzymes: Their diversity and emerging roles. Biochem. Biophys. Res. Commun. 266, 633–640 (1999)
D'Andrea, A. & Pellman, D. Deubiquitinating enzymes: A new class of biological regulators. Crit. Rev. Biochem. Mol. Biol. 33, 337–352 (1998)
Bignell, G. R. et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nature Genet. 25, 160–165 (2000)
Yin, M. J., Yamamoto, Y. & Gaynor, R. B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature 396, 77–80 (1998)
Nakamura, T., Hillova, J., Mariage-Samson, R. & Hill, M. Molecular cloning of a novel oncogene generated by DNA recombination during transfection. Oncogene Res. 2, 357–370 (1988)
Papa, F. R. & Hochstrasser, M. The yeast DOA4 gene encodes a deubiquitinating enzyme related to a product of the human tre-2 oncogene. Nature 366, 313–319 (1993)
Brummelkamp, T. R., Bernards, R. & Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553 (2002)
Karin, M., Cao, Y., Greten, F. R. & Li, Z. W. NF-κB in cancer: From innocent bystander to major culprit. Nature Rev. Cancer 2, 301–310 (2002)
Smahi, A. et al. The NF-κB signalling pathway in human diseases: From incontinentia pigmenti to ectodermal dysplasias and immune-deficiency syndromes. Hum. Mol. Genet. 11, 2371–2375 (2002)
Trompouki, E. et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-kB activation by TNFR family members. Nature 424, 793–796 (2003)
Bradley, J. R. & Pober, J. S. Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20, 6482–6491 (2001)
Chung, J. Y., Park, Y. C., Ye, H. & Wu, H. All TRAFs are not created equal: Common and distinct molecular mechanisms of TRAF-mediated signal transduction. J. Cell Sci. 115, 679–688 (2002)
Shi, C. S. & Kehrl, J. H. TNF-induced GCKR and SAPK activation depends upon the E2/E3 complex Ubc13-Uev1A/TRAF2. J. Biol. Chem. 18, 15429–15434 (2003)
Wang, C. et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351 (2001)
Holtmann, H., Hahn, T. & Wallach, D. Interrelated effects of tumor necrosis factor and interleukin 1 on cell viability. Immunobiology 177, 7–22 (1988)
Wang, C. Y., Mayo, M. W. & Baldwin, A. S. Jr TNF- and cancer therapy-induced apoptosis: Potentiation by inhibition of NF-κB. Science 274, 784–787 (1996)
Liu, Z. G., Hsu, H., Goeddel, D. V. & Karin, M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 87, 565–576 (1996)
De Smaele, E. et al. Induction of gadd45β by NF-κB downregulates pro-apoptotic JNK signalling. Nature 414, 308–313 (2001)
Baud, V. & Karin, M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 11, 372–377 (2001)
Rossi, A. et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature 403, 103–108 (2000)
Dajee, M. et al. NF-κB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature 421, 639–643 (2003)
van Balkom, I. D. & Hennekam, R. C. Dermal eccrine cylindromatosis. J. Med. Genet. 31, 321–324 (1994)
Schmidt-Ullrich, R. et al. NF-κB activity in transgenic mice: Developmental regulation and tissue specificity. Development 122, 2117–2128 (1996)
Schmidt-Ullrich, R. et al. Requirement of NF-κB/Rel for the development of hair follicles and other epidermal appendices. Development 128, 3843–3853 (2001)
Agami, R. & Bernards, R. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell 102, 55–66 (2000)
Chen, G., Cao, P. & Goeddel, D. V. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol. Cell 9, 401–410 (2002)
Acknowledgements
We thank S. Lens, A. Lund and J. Borst for reagents, and M. Madiredjo for assistance. This work was supported by the Centre for Biomedical Genetics (CBG) and the Netherlands Organization for Scientific Research (NWO). A.D. was supported by a long-term fellowship from EMBO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Brummelkamp, T., Nijman, S., Dirac, A. et al. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-κB. Nature 424, 797–801 (2003). https://doi.org/10.1038/nature01811
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01811