Abstract
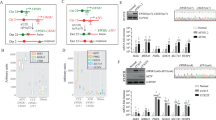
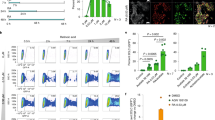
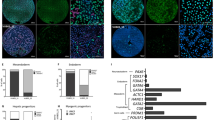
Embryonic stem (ES) cells are pluripotent cells derived from early mammalian embryos1,2. Their immortality and rapid growth make them attractive sources for stem cell therapies3; however, they produce tumours (teratomas) when transplanted, which could preclude their therapeutic usage4. Why ES cells, which lack chromosomal abnormalities, possess tumour-like properties is largely unknown. Here we show that mouse ES cells specifically express a Ras-like gene, which we have named ERas. We show that human HRasp, which is a recognized pseudogene, does not contain reported base substitutions and instead encodes the human orthologue of ERas. This protein contains amino-acid residues identical to those present in active mutants of Ras5 and causes oncogenic transformation in NIH 3T3 cells. ERas interacts with phosphatidylinositol-3-OH kinase6 but not with Raf7,8. ERas-null ES cells maintain pluripotency but show significantly reduced growth and tumorigenicity, which are rescued by expression of ERas complementary DNA or by activated phosphatidylinositol-3-OH kinase. We conclude that the transforming oncogene ERas is important in the tumour-like growth properties of ES cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Evans, M. J. & Kaufman, M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 (1981)
Martin, G. R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA 78, 7634–7638 (1981)
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998)
Freed, C. R. Will embryonic stem cells be a useful source of dopamine neurons for transplant into patients with Parkinson's disease? Proc. Natl Acad. Sci. USA 99, 1755–1757 (2002)
Seeburg, P. H., Colby, W. W., Capon, D. J., Goeddel, D. V. & Levinson, A. D. Biological properties of human c-Ha-ras1 genes mutated at codon 12. Nature 312, 71–75 (1984)
Rodriguez-Viciana, P. et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370, 527–532 (1994)
Moodie, S. A., Willumsen, B. M., Weber, M. J. & Wolfman, A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science 260, 1658–1661 (1993)
Zhang, X. F. et al. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature 364, 308–313 (1993)
Takai, Y., Sasaki, T. & Matozaki, T. Small GTP-binding proteins. Physiol. Rev. 81, 153–208 (2001)
Chen, Z. Q., Ulsh, L. S., DuBois, G. & Shih, T. Y. Posttranslational processing of p21 ras proteins involves palmitylation of the C-terminal tetrapeptide containing cysteine-186. J. Virol. 56, 607–612 (1985)
Willumsen, B. M., Christensen, A., Hubbert, N. L., Papageorge, A. G. & Lowy, D. R. The p21 ras C-terminus is required for transformation and membrane association. Nature 310, 583–586 (1984)
Miyoshi, J., Kagimoto, M., Soeda, E. & Sakaki, Y. The human c-Ha-ras2 is a processed pseudogene inactivated by numerous base substitutions. Nucleic Acids Res. 12, 1821–1828 (1984)
Meiner, V. L. et al. Disruption of the acyl-CoA:cholesterol acyltransferase gene in mice: evidence suggesting multiple cholesterol esterification enzymes in mammals. Proc. Natl Acad. Sci. USA 93, 14041–14046 (1996)
Li, E., Bestor, T. H. & Jaenisch, R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69, 915–926 (1992)
Nichols, J., Evans, E. P. & Smith, A. G. Establishment of germ-line-competent embryonic stem (ES) cells using differentiation inhibiting activity. Development 110, 1341–1348 (1990)
Gassmann, M., Donoho, G. & Berg, P. Maintenance of an extrachromosomal plasmid vector in mouse embryonic stem cells. Proc. Natl Acad. Sci. USA 92, 1292–1296 (1995)
Fasano, O. et al. Analysis of the transforming potential of the human H-ras gene by random mutagenesis. Proc. Natl Acad. Sci. USA 81, 4008–4012 (1984)
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997)
Cheng, A. M. et al. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell 95, 793–803 (1998)
Burdon, T., Stracey, C., Chambers, I., Nichols, J. & Smith, A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev. Biol. 210, 30–43 (1999)
Rodriguez-Viciana, P. et al. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell 89, 457–467 (1997)
Di Cristofano, A., Pesce, B., Cordon-Cardo, C. & Pandolfi, P. P. Pten is essential for embryonic development and tumour suppression. Nature Genet. 19, 348–355 (1998)
Sun, H. et al. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5-trisphosphate and Akt/protein kinase B signaling pathway. Proc. Natl Acad. Sci. USA 96, 6199–6204 (1999)
Burgering, B. M. & Coffer, P. J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376, 599–602 (1995)
Franke, T. F. et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81, 727–736 (1995)
Klippel, A. et al. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol. Cell. Biol. 16, 4117–4127 (1996)
Jirmanova, L., Afanassieff, M., Gobert-Gosse, S., Markossian, S. & Savatier, P. Differential contributions of ERK and PI3-kinase to the regulation of cyclin D1 expression and to the control of the G1/S transition in mouse embryonic stem cells. Oncogene 21, 5515–5528 (2002)
Quilliam, L. A. M.-R. et al. Ras/R-Ras3, a transforming ras protein regulated by Sos1, GRF1, and p120 Ras GTPase-activating protein, interacts with the putative Ras effector AF6. J. Biol. Chem. 274, 23850–23857 (1999)
Clark, G. J., Cox, A. D., Graham, S. M. & Der, C. J. Biological assays for Ras transformation. Methods Enzymol. 255, 395–412 (1995)
Rosario, M., Paterson, H. F. & Marshall, C. J. Activation of the Raf/MAP kinase cascade by the Ras-related protein TC21 is required for the TC21-mediated transformation of NIH 3T3 cells. EMBO J. 18, 1270–1279 (1999)
Acknowledgements
We thank E. Kaiho, Y. Tokuzawa and M. Murakami for discussion; C. Takigawa and J. Iida for technical assistance; T. Ichisaka and Y. Samitsu for blastocyst microinjection; R. Farese Jr for RF8 ES cells, R. Jaenisch and T. Noda for J1 cells; W. Skarnes and S. Young for CGR8 cells; H. Niwa for MG1.19 cells, pCAG-IP and pBIKS(- )BgeopA; M. Okabe and J.-i. Miyazaki for pCX–EGFP; K. Kohno and T. Kitamura for PLAT-E cells and pMX retroviral vectors; and S. Young, R. Farese Jr and R. Pitas for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Takahashi, K., Mitsui, K. & Yamanaka, S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature 423, 541–545 (2003). https://doi.org/10.1038/nature01646
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01646