Abstract
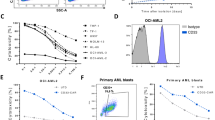
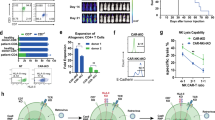
Chimeric antigen receptors (CARs) have been used to redirect the specificity of autologous T cells against leukemia and lymphoma with promising clinical results. Extending this approach to allogeneic T cells is problematic as they carry a significant risk of graft-versus-host disease (GVHD). Natural killer (NK) cells are highly cytotoxic effectors, killing their targets in a non-antigen-specific manner without causing GVHD. Cord blood (CB) offers an attractive, allogeneic, off-the-self source of NK cells for immunotherapy. We transduced CB-derived NK cells with a retroviral vector incorporating the genes for CAR-CD19, IL-15 and inducible caspase-9-based suicide gene (iC9), and demonstrated efficient killing of CD19-expressing cell lines and primary leukemia cells in vitro, with marked prolongation of survival in a xenograft Raji lymphoma murine model. Interleukin-15 (IL-15) production by the transduced CB-NK cells critically improved their function. Moreover, iC9/CAR.19/IL-15 CB-NK cells were readily eliminated upon pharmacologic activation of the iC9 suicide gene. In conclusion, we have developed a novel approach to immunotherapy using engineered CB-derived NK cells, which are easy to produce, exhibit striking efficacy and incorporate safety measures to limit toxicity. This approach should greatly improve the logistics of delivering this therapy to large numbers of patients, a major limitation to current CAR-T-cell therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sadelain M, Riviere I, Brentjens R . Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer 2003; 3: 35–45.
Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME . Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008; 8: 299–308.
June CH, Blazar BR, Riley JL . Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol 2009; 9: 704–716.
Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5: 177ra38.
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368: 1509–1518.
Porter DL, Levine BL, Kalos M, Bagg A, June CH . Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011; 365: 725–733.
Goulmy E . Human minor histocompatibility antigens: new concepts for marrow transplantation and adoptive immunotherapy. Immunol Rev 1997; 157: 125–140.
Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295: 2097–2100.
Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol 2010; 28: 955–959.
Caligiuri MA, Velardi A, Scheinberg DA, Borrello IM . Immunotherapeutic approaches for hematologic malignancies. Hematology Am Soc Hematol Educ Program 2004, 337–353.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385: 517–528.
Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005; 105: 3051–3057.
Curti A, Ruggeri L, D'Addio A, Bontadini A, Dan E, Motta MR et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood 2011; 118: 3273–3279.
Rouce RH, Shaim H, Sekine T, Weber G, Ballard B, Ku S et al. The TGF-beta/SMAD pathway is an important mechanism for NK cell immune evasion in childhood B-acute lymphoblastic leukemia. Leukemia 2016; 30: 800–811.
Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica 2014; 99: 836–847.
Shah N, Martin-Antonio B, Yang H, Ku S, Lee DA, Cooper LJ et al. Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PLoS One 2013; 8: e76781.
Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 2009; 69: 4010–4017.
Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ . Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics 2016; 3: 16011.
Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 2010; 24: 1160–1170.
Tagaya Y, Bamford RN, DeFilippis AP, Waldmann TA . IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity 1996; 4: 329–336.
Di SA, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 2011; 365: 1673–1683.
Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE 2012; 7: e30264.
Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood 2006; 108: 3890–3897.
Kruse V, Hamann C, Monecke S, Cyganek L, Elsner L, Hubscher D et al. Human induced pluripotent stem cells are targets for allogeneic and autologous natural killer (NK) cells and killing is partly mediated by the activating NK receptor DNAM-1. PLoS ONE 2015; 10: e0125544.
Sanborn KB, Rak GD, Mentlik AN, Banerjee PP, Orange JS . Analysis of the NK cell immunological synapse. Methods Mol Biol 2010; 612: 127–148.
Cany J, van der Waart AB, Tordoir M, Franssen GM, Hangalapura BN, de Vries J et al. Natural killer cells generated from cord blood hematopoietic progenitor cells efficiently target bone marrow-residing human leukemia cells in NOD/SCID/IL2Rg(null) mice. PLoS One 2013; 8: e64384.
Chen X, Allan DS, Krzewski K, Ge B, Kopcow H, Strominger JL . CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc Natl Acad Sci USA 2006; 103: 10346–10351.
Gill S, Vasey AE, De SA, Baker J, Smith AT, Kohrt HE et al. Rapid development of exhaustion and down-regulation of eomesodermin limit the antitumor activity of adoptively transferred murine natural killer cells. Blood 2012; 119: 5758–5768.
Elpek KG, Rubinstein MP, Bellemare-Pelletier A, Goldrath AW, Turley SJ . Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Ralpha complexes. Proc Natl Acad Sci USA 2010; 107: 21647–21652.
Lanier LL . Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008; 9: 495–502.
Lanier LL . On guard—activating NK cell receptors. Nat Immunol 2001; 2: 23–27.
Schwartz RH . Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell 1992; 71: 1065–1068.
Lanier LL, O'Fallon S, Somoza C, Phillips JH, Linsley PS, Okumura K et al. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol 1995; 154: 97–105.
Azuma M, Cayabyab M, Buck D, Phillips JH, Lanier LL . Involvement of CD28 in MHC-unrestricted cytotoxicity mediated by a human natural killer leukemia cell line. J Immunol 1992; 149: 1115–1123.
Galea-Lauri J, Darling D, Gan SU, Krivochtchapov L, Kuiper M, Gaken J et al. Expression of a variant of CD28 on a subpopulation of human NK cells: implications for B7-mediated stimulation of NK cells. J Immunol 1999; 163: 62–70.
Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 2010; 116: 2411–2419.
Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood 2009; 113: 726–732.
Sekine T, Marin D, Cao K, Li L, Mehta P, Shaim H et al. Specific combinations of donor and recipient KIR-HLA genotypes predict for large differences in outcome after cord blood transplantation. Blood 2016; 128: 297–312.
Wang JW, Howson JM, Ghansah T, Desponts C, Ninos JM, May SL et al. Influence of SHIP on the NK repertoire and allogeneic bone marrow transplantation. Science 2002; 295: 2094–2097.
Zhang Y, Wallace DL, de Lara CM, Ghattas H, Asquith B, Worth A et al. in vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology 2007; 121: 258–265.
Sun JC, Beilke JN, Lanier LL . Adaptive immune features of natural killer cells. Nature 2009; 457: 557–561.
Sun JC, Beilke JN, Bezman NA, Lanier LL . Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J Exp Med 2011; 208: 357–368.
Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 2010; 116: 3865–3874.
Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol 2012; 189: 5082–5088.
Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL . NK cells and immune ‘memory’. J Immunol 2011; 186: 1891–1897.
Corat MA, Schlums H, Wu C, Theorell J, Espinoza DA, Sellers SE et al. Acquired somatic mutations in PNH reveal long-term maintenance of adaptive NK cells independent of HSPCs. Blood 2017; 129: 1940–1946.
Tesi B, Davidsson J, Voss M, Rahikkala E, Holmes TD, Chiang SCC et al. Gain-of-function SAMD9L mutations cause a syndrome of cytopenia, immunodeficiency, MDS, and neurological symptoms. Blood 2017; 129: 2266–2279.
Dudley ME, Rosenberg SA . Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer 2003; 3: 666–675.
Sahm C, Schonfeld K, Wels WS . Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified effectors that carry a tumor-specific antigen receptor. Cancer Immunol Immunother 2012; 61: 1451–1461.
Mishra A, Liu S, Sams GH, Curphey DP, Santhanam R, Rush LJ et al. Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. Cancer Cell 2012; 22: 645–655.
Nellan A, Lee DW . Paving the road ahead for CD19 CAR T-cell therapy. Curr Opin Hematol 2015; 22: 516–520.
Acknowledgements
This work was funded in part by LLS 6470-15, ACS RSG-15-218-01-LIB and the generous philanthropic contributions to The University of Texas MD Anderson Moon Shots Program. The flow studies were performed in the Flow Cytometry & Cellular Imaging Facility, which is supported in part by the National Institutes of Health through MD Anderson Cancer Center Support Grant CA016672.
Author contributions
EL designed and performed experiments, interpreted the data and wrote the manuscript, YT, MaM, HS, XL, AR, MG, LL, MHB, XW, RC, RB and PB performed experiments and commented on the manuscript. GP, BS, MuM, JO, MK, DM, WW, RC and EJS provided advice on experiments and commented on the manuscript. KR designed and directed the study and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Liu, E., Tong, Y., Dotti, G. et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 32, 520–531 (2018). https://doi.org/10.1038/leu.2017.226
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.226