Abstract
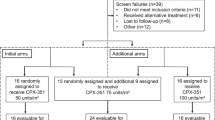
Heat shock protein 90 (Hsp90) is a molecular chaperone with many oncogenic client proteins. The small-molecule Hsp90 inhibitor alvespimycin, a geldanamycin derivative, is being developed for various malignancies. This phase 1 study examined the maximum-tolerated dose (MTD), safety and pharmacokinetic/pharmacodynamic profiles of alvespimycin in patients with advanced acute myeloid leukemia (AML). Patients with advanced AML received escalating doses of intravenous alvespimycin (8–32 mg/m2), twice weekly, for 2 of 3 weeks. Dose-limiting toxicities (DLTs) were assessed during cycle 1. A total of 24 enrolled patients were evaluable for toxicity. Alvespimycin was well tolerated; the MTD was 24 mg/m2 twice weekly. Common toxicities included neutropenic fever, fatigue, nausea and diarrhea. Cardiac DLTs occurred at 32 mg/m2 (elevated troponin and myocardial infarction). Pharmacokinetics revealed linear increases in Cmax and area under the curve (AUC) from 8 to 32 mg/m2 and minor accumulation upon repeated doses. Pharmacodynamic analyses on day 15 revealed increased apoptosis and Hsp70 levels when compared with baseline within marrow blasts. Antileukemia activity occurred in 3 of 17 evaluable patients (complete remission with incomplete blood count recovery). The twice-weekly administered alvespimycin was well tolerated in patients with advanced AML, showing linear pharmacokinetics, target inhibition and signs of clinical activity. We determined a recommended phase 2 dose of 24 mg/m2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Powers MV, Workman P . Inhibitors of the heat shock response: biology and pharmacology. FEBS Lett 2007; 581: 3758–3769.
Nimmanapalli R, O’Bryan E, Bhalla K . Geldanamycin and its analogue 17-allylamino-17-demethoxygeldanamycin lowers Bcr-Abl levels and induces apoptosis and differentiation of Bcr-Abl-positive human leukemic blasts. Cancer Res 2001; 61: 1799–1804.
Pearl LH, Prodromou C, Workman P . The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J 2008; 410: 439–453.
Whitesell L, Lindquist SL . HSP90 and the chaperoning of cancer. Nat Rev Cancer 2005; 5: 761–772.
Solit DB, Ivy SP, Kopil C, Sikorski R, Morris MJ, Slovin SF et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. Clin Cancer Res 2007; 13: 1775–1782.
Ramanathan RK, Egorin MJ, Eiseman JL, Ramalingam S, Friedland D, Agarwala SS et al. Phase I and pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with refractory advanced cancers. Clin Cancer Res 2007; 13: 1769–1774.
Kaur G, Belotti D, Burger AM, Fisher-Nielson K, Borsotti P, Riccardi E et al. Antiangiogenic properties of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin: an orally bioavailable heat shock protein 90 modulator. Clin Cancer Res 2004; 10: 4813–4821.
Smith V, Sausville EA, Camalier RF, Fiebig HH, Burger AM . Comparison of 17-dimethylaminoethylamino-17-demethoxy-geldanamycin (17DMAG) and 17-allylamino-17-demethoxygeldanamycin (17AAG) in vitro: effects on Hsp90 and client proteins in melanoma models. Cancer Chemother Pharmacol 2005; 56: 126–137.
Murgo AJ, Kummar S, Gardner ER . Phase I trial of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) administered twice weekly [abstract]. J Clin Oncol 2007; 25: 3566.
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2003; 21: 4642–4649.
Faderl S, Talpaz M, Estrov Z, Kantarjian HM . Chronic myelogenous leukemia: biology and therapy. Ann Intern Med 1999; 131: 207–219.
Bareng J, Jilani I, Gorre M, Kantarjian H, Giles F, Hannah A et al. A potential role for HSP90 inhibitors in the treatment of JAK2 mutant-positive diseases as demonstrated using quantitative flow cytometry. Leuk Lymphoma 2007; 48: 2189–2195.
Banerji U, O’Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y et al. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol 2005; 23: 4152–4161.
Grandage VL, Linch DC, Khwaja A . PI3 kinase is constitutively active in primary acute myeloid leukaemias and regulates survival and chemoresistance via NFkB, MAP Kinase and p53 pathways [abstract]. Blood 2003; 102: 2156.
Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood 2006; 108: 2358–2365.
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 2001; 98: 1752–1759.
Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood 2002; 100: 59–66.
Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002; 99: 4326–4335.
Towatari M, Iida H, Iwata H, Hamaguchi M, Saito H . Constitutive activation of mitogen-activated protein kinase pathway in acute leukemia cells. Leukemia 1997; 11: 479–484.
Cairoli R, Cairoli R, Beghini A, Grillo G, Nadali G, Elice F et al. Prognostic impact of c-KIT mutations in core binding factor leukemias: an Italian retrospective study. Blood 2006; 107: 3463–3468.
Paschka P, Marcucci G, Ruppert AS, Mrózek K, Chen H, Kittles RA et al. Adverse prognostic significance of kit mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol 2006; 24: 3904–3911.
Schnittger S, Kohl TM, Haferlach T, Kern W, Hiddemann W, Spiekermann K et al. KIT-D816 mutations in AML1-ETO-positive AML are associated with impaired event-free and overall survival. Blood 2006; 107: 1791–1799.
Thomas X, Campos L, Le QH, Guyotat D . Heat shock proteins and acute leukemias. Hematology 2005; 10: 225–235.
Ferrarini M, Heltai S, Zocchi MR, Rugarli C . Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer 1992; 51: 613–619.
Piloto O, Wright M, Brown P, Kim KT, Levis M, Small D . Prolonged exposure to FLT3 inhibitors leads to resistance via activation of parallel signaling pathways. Blood 2007; 109: 1643–1652.
Acknowledgements
This work was supported by Kosan Biosciences, Hayward, CA, USA.
We thank Michelle Mintz, ARNP, for her excellent care of the patients. We also thank Rasa Hamilton for assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
MX Albitar and AL Hannah are former Kosan consultants (compensated), and K Kersey and Z Zhong are former Kosan employees.
Additional information
Presented in abstract form at the 48th annual meeting of the American Society of Hematology, Orlando, FL, 10 December 2006.
Rights and permissions
About this article
Cite this article
Lancet, J., Gojo, I., Burton, M. et al. Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia 24, 699–705 (2010). https://doi.org/10.1038/leu.2009.292
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2009.292