Abstract
Aims:
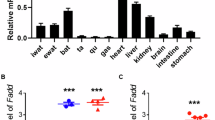
The objective of this study is to characterize the relationship between forkhead box C2 protein (Foxc2) and leptin under adipose inflammatory response.
Methods:
Lipopolysaccharide (LPS)-induced inflammatory model was conducted. Data from wild-type and ob/ob mice were used to compare the alternative role of leptin on Foxc2-mediated inflammation and browning. Transcriptional regulation and protein-protein interaction were analyzed by bioinformatics and proved by chromatin immunoprecipitation and co-immunoprecipitation experiment.
Results:
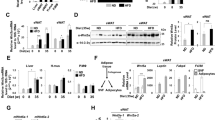
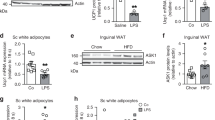
Foxc2 and leptin correlated with inflammation and browning of white adipose tissue (WAT) in LPS-treated mice. Moreover, Foxc2-mediated inhibition of inflammation involved downstream activation of leptin signal and promoted WAT browning. We then determined CREB, the potential transcriptional factor of leptin, was required for Foxc2-mediated inflammation in the regulation of WAT browning. Foxc2 alleviated adipocyte inflammation by reducing leptin-mediated Janus-activated kinase 2/signal transducer and activator of transcription 3 (STAT3) pathway. Importantly, STAT3 physically interacted with PRDM16 and formed a complex to promote WAT browning. Exogenous Foxc2 overexpression also ameliorated inflammation and promoted adipose browning in high fat diet (HFD)-induced obese mice.
Conclusions:
Our results indicated that Foxc2 inhibited inflammation and promoted browning of WAT through positive regulation of leptin signal and the STAT3-PRDM16 complex. These findings identify a new potential means to prevent and treat obese caused metabolic syndrome of mammals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Henao-Mejia J, Elinav E, Thaiss CA, Flavell RA . Inflammasomes and metabolic disease. Annu Rev Physiol 2014; 76: 57–78.
van der Heijden RA, Sheedfar F, Morrison MC, Hommelberg PP, Kor D, Kloosterhuis NJ et al. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging 2015; 7: 256–268.
Kusminski CM, Bickel PE, Scherer PE . Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat Rev Drug Discov 2016; 15: 639–660.
Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 2016; 23: 591–601.
Peirce V, Carobbio S, Vidal-Puig A . The different shades of fat. Nature 2014; 510: 76–83.
Cannon B, Nedergaard J . Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84: 277–359.
Cinti S . Adipocyte differentiation and transdifferentiation: plasticity of the adipose organ. J Endocrinol Invest 2002; 25: 823–835.
Ohno H, Shinoda K, Spiegelman BM, Kajimura S . PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 2012; 15: 395–404.
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC et al. A PGC1-[agr]-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481: 463–468.
Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab 2011; 14: 324–338.
Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 2011; 121: 96.
Fruhbeck G . Intracellular signalling pathways activated by leptin. Biochem J 2006; 393: 7–20.
Rodríguez A . Novel molecular aspects of ghrelin and leptin in the control of adipobiology and the cardiovascular system. Obes Facts 2014; 7: 82–95.
Commins SP, Watson PM, Levin N, Beiler RJ, Gettys TW . Central leptin regulates the UCP1 and obGenes in brown and white adipose tissue via different β-adrenoceptor subtypes. J Biol Chem 2000; 275: 33059–33067.
Morrison SF, Madden CJ, Tupone D . Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 2014; 19: 741–756.
Myers MG, Cowley MA, Münzberg H . Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 2008; 70: 537–556.
Moisan A, Lee YK, Zhang JD, Hudak CS, Meyer CA, Prummer M et al. White-to-brown metabolic conversion of human adipocytes by JAK inhibition. Nat Cell Biol 2015; 17: 57–67.
Luan B, Goodarzi MO, Phillips NG, Guo X, Chen YD, Yao J et al. Leptin-mediated increases in catecholamine signaling reduce adipose tissue inflammation via activation of macrophage HDAC4. Cell Metab 2014; 19: 1058–1065.
Arteaga-Solis E, Zee T, Emala CW, Vinson C, Wess J, Karsenty G . Inhibition of leptin regulation of parasympathetic signaling as a cause of extreme body weight-associated asthma. Cell Metab 2013; 17: 35–48.
Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA et al. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 2015; 132: 2134–2145.
Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab 2014; 20: 103–118.
Liu Z, Gan L, Zhou Z, Jin W, Sun C . SOCS3 promotes inflammation and apoptosis via inhibiting JAK2/STAT3 signaling pathway in 3T3-L1 adipocyte. Immunobiology 2015; 220: 947–953.
Lidell ME, Seifert EL, Westergren R, Heglind M, Gowing A, Sukonina V et al. The adipocyte-expressed forkhead transcription factor Foxc2 regulates metabolism through altered mitochondrial function. Diabetes 2011; 60: 427–435.
Gan L, Liu Z, Jin W, Zhou Z, Sun C . Foxc2 enhances proliferation and inhibits apoptosis through activating Akt/mTORC1 signaling pathway in mouse preadipocytes. J Lipid Res 2015; 56: 1471–1480.
Cederberg A, Grønning LM, Ahrén B, Taskén K, Carlsson P, Enerbäck S . FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 2001; 106: 563–573.
Rahman S, Lu Y, Czernik PJ, Rosen CJ, Enerback S, Lecka-Czernik B . Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology 2013; 154: 2687–2701.
Kim JK, Kim H-J, Park S-Y, Cederberg A, Westergren R, Nilsson D et al. Adipocyte-specific overexpression of FOXC2 prevents diet-induced increases in intramuscular fatty acyl CoA and insulin resistance. Diabetes 2005; 54: 1657–1663.
Li X, Massa PE, Hanidu A, Peet GW, Aro P, Savitt A et al. IKKalpha, IKKbeta, and NEMO/IKKgamma are each required for the NF-kappa B-mediated inflammatory response program. J Biol Chem 2002; 277: 45129–45140.
Nedergaard J, Cannon B . The browning of white adipose tissue: some burning issues. Cell Metab 2014; 20: 396–407.
Véniant MM, Sivits G, Helmering J, Komorowski R, Lee J, Fan W et al. Pharmacologic effects of FGF21 are independent of the “browning” of white adipose tissue. Cell Metab 2015; 21: 731–738.
Kumari M, Wang X, Lantier L, Lyubetskaya A, Eguchi J, Kang S et al. IRF3 promotes adipose inflammation and insulin resistance and represses browning. J Clin Invest 2016; 126: 2839–2854.
Shen W, Chuang CC, Martinez K, Reid T, Brown JM, Xi L et al. Conjugated linoleic acid reduces adiposity and increases markers of browning and inflammation in white adipose tissue of mice. J Lipid Res 2013; 54: 909–922.
Gan L, Liu Z, Chen Y, Dan L, Feng F, Liu G et al. alpha-MSH and Foxc2 promote fatty acid oxidation through C/EBPbeta negative transcription in mice adipose tissue. Sci Rep 2016; 6: 36661.
Friedman JM, Halaas JL . Leptin and the regulation of body weight in mammals. Nature 1998; 395: 763–770.
Zhou Z, Neupane M, Zhou HR, Wu D, Chang C-C, Moustaid-Moussa N et al. Leptin differentially regulate STAT3 activation in ob/ob mouse adipose mesenchymal stem cells. Nutr Metab 2012; 9: 109.
Kanda Y, Hinata T, Kang SW, Watanabe Y . Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci 2011; 89: 250–258.
Dempersmier J, Sambeat A, Gulyaeva O, Paul SM, Hudak CS, Raposo HF et al. Cold-inducible Zfp516 activates UCP1 transcription to promote browning of white fat and development of brown fat. Mol Cell 2015; 57: 235–246.
Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, Eizirik DL et al. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation 2001; 104: 979–981.
Zhang K, Guo W, Yang Y, Wu J . JAK2/STAT3 pathway is involved in the early stage of adipogenesis through regulating C/EBPβ transcription. J Cell Biochem 2011; 112: 488–497.
Wang D, Zhou Y, Lei W, Zhang K, Shi J, Hu Y et al. Signal transducer and activator of transcription 3 (STAT3) regulates adipocyte differentiation via peroxisome-proliferator-activated receptor γ (PPARγ). Biol Cell 2010; 102: 1–12.
Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA 2004; 101: 4661–4666.
Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG, Rossetti L . Critical role of STAT3 in leptin's metabolic actions. Cell Metab 2006; 4: 49–60.
Piper ML, Unger EK, Myers MG Jr, Xu AW . Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol Endocrinol 2008; 22: 751–759.
Acknowledgements
This work was supported by the Major National Scientific Research Projects under Grant 2015CB943102 and the National Nature Science Foundation of China under Grant 31572365.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Gan, L., Liu, Z., Feng, F. et al. Foxc2 coordinates inflammation and browning of white adipose by leptin-STAT3-PRDM16 signal in mice. Int J Obes 42, 252–259 (2018). https://doi.org/10.1038/ijo.2017.208
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.208
This article is cited by
-
Cissus Quadrangularis enhances UCP1 mRNA, indicative of white adipocyte browning and decreases central obesity in humans in a randomized trial
Scientific Reports (2021)
-
Testosterone treatment is associated with reduced adipose tissue dysfunction and nonalcoholic fatty liver disease in obese hypogonadal men
Journal of Endocrinological Investigation (2021)
-
Impact of adipokines and myokines on fat browning
Journal of Physiology and Biochemistry (2020)
-
Foxc2 Alleviates Ox-LDL-Induced Lipid Accumulation, Inflammation, and Apoptosis of Macrophage via Regulating the Expression of Angptl2
Inflammation (2020)
-
The gut microbiota modulates both browning of white adipose tissue and the activity of brown adipose tissue
Reviews in Endocrine and Metabolic Disorders (2019)