Abstract
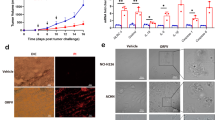
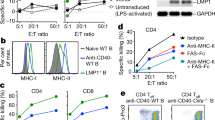
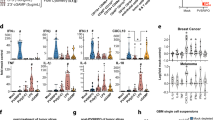
Oncolytic viruses (OV) are promising treatments for cancer, with several currently undergoing testing in randomised clinical trials. Measles virus (MV) has not yet been tested in models of human melanoma. This study demonstrates the efficacy of MV against human melanoma. It is increasingly recognised that an essential component of therapy with OV is the recruitment of host antitumour immune responses, both innate and adaptive. MV-mediated melanoma cell death is an inflammatory process, causing the release of inflammatory cytokines including type-1 interferons and the potent danger signal HMGB1. Here, using human in vitro models, we demonstrate that MV enhances innate antitumour activity, and that MV-mediated melanoma cell death is capable of stimulating a melanoma-specific adaptive immune response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Grote D, Russell SJ, Cornu TI, Cattaneo R, Vile R, Poland GA et al. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood 2001; 97: 3746–3754.
Peng KW, Ahmann GJ, Pham L, Greipp PR, Cattaneo R, Russell SJ et al. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood 2001; 98: 2002–2007.
Peng K-W, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC, Russell SJ et al. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res 2002; 62: 4656–4662.
Phuong LK, Allen C, Peng KW, Giannini C, Greiner S, TenEyck CJ et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res 2003; 63: 2462–2469.
Peng K-W, Frenzke M, Myers R, Soeffker D, Harvey M, Greiner S et al. Biodistribution of oncolytic measles virus after intraperitoneal administration into Ifnar-CD46Ge transgenic mice. Hum Gene Ther 2003; 14: 1565–1577.
Dingli D, Dingli D, Peng KW, Harvey ME, Greipp PR, O’Connor MK et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood 2004; 103: 1641–1646.
Langfield KK, Wegman TR, Walker HJ, Griesmann GE, Sauer JA, Stephan SA et al. 77. Production and purification of measles virus for oncolytic virotherapy clinical trials. Mol Ther 2004; 9: S31.
Kunzi V, Oberholzer PA, Heinzerling L, Dummer R, Naim HY . Recombinant measles virus induces cytolysis of cutaneous T-cell lymphoma in vitro and in vivo. J Invest Dermatol 2006; 126: 2525–2532.
Hasegawa K, Pham L, O’Connor MK, Federspiel MJ, Russell SJ, Peng KW et al. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin Cancer Res 2006; 12: 1868–1875.
Blechacz B, Splinter PL, Greiner S, Myers R, Peng KW, Federspiel MJ et al. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology 2006; 44: 1465–1477.
Zimmermann M, Armeanu S, Smirnow I, Kupka S, Wagner S, Wehrmann M et al. Human precision-cut liver tumor slices as a tumor patient-individual predictive test system for oncolytic measles vaccine viruses. Int J Oncol 2009; 34: 1247–1256.
Penheiter AR, Wegman TR, Classic KL, Dingli D, Bender CE, Russell SJ et al. Sodium iodide symporter (NIS)-mediated radiovirotherapy for pancreatic cancer. Am J Roentgenol 2010; 195: 341–349.
Heinzerling L, Künzi V, Oberholzer PA, Kündig T, Naim H, Dummer R et al. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood 2005; 106: 2287–2294.
Msaouel P, Dispenzieri A, Galanis E . Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview. Curr Opin Mol Ther 2009; 11: 43–53.
Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol 2010; 17: 718–730.
Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 2009; 27: 5763–5771.
O’Day S, Hodi FS, McDermott DF, Weber RW, Sosman JA, Haanen JB et al. A phase III, randomized, double-blind, multicenter study comparing monotherapy with ipilimumab or gp100 peptide vaccine and the combination in patients with previously treated, unresectable stage III or IV melanoma. J Clin Oncol 2010; 28: 4.
Gauvrit A, Brandler S, Sapede-Peroz C, Boisgerault N, Tangy F, Gregoire M et al. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res 2008; 68: 4882–4892.
Kemper C, Atkinson JP . Measles virus and CD46. Curr Top Microbiol Immunol 2009; 329: 31–57.
Anderson BD, Nakamura T, Russell SJ, Peng K-WHighCD46 . Receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res 2004; 64: 4919–4926.
Donnelly OG, Errington-Mais F, Prestwich R, Harrington K, Pandha H, Vile R et al. Recent clinical experience with oncolytic viruses. Curr Pharm Biotechnol 2011, e-pub ahead of print 8 July 2011. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21740364. ISN 1873-4316.
Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res 2010; 70: 875–882.
Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther 2009; 20: 1119–1132.
Wongthida P, Diaz RM, Galivo F, Kottke T, Thompson J, Melcher A et al. VSV oncolytic virotherapy in the B16 model depends upon intact MyD88 signaling. Mol Ther 2011; 19: 150–158.
Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res 2009; 15: 4374–4381.
Errington F, Steele L, Prestwich R, Harrington KJ, Pandha HS, Vidal L et al. Reovirus activates human dendritic cells to promote innate antitumor immunity. J Immunol 2008; 180: 6018–6026.
Prestwich RJ, Errington F, Ilett EJ, Morgan RS, Scott KJ, Kottke T et al. Tumor Infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res 2008; 14: 7358–7366.
Reed LJ, Muench H . A simple method of estimating fifty per cent endpoints. Am J Epidemiol 1938; 27: 493.
Lee GY, Kenny PA, Lee EH, Bissell MJ . Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Meth 2007; 4: 359–365.
Evans CJ, Phillips RM, Jones PF, Loadman PM, Sleeman BD, Twelves CJ et al. A mathematical model of doxorubicin penetration through multicellular layers. J Theor Biol 2009; 257: 598–608.
Errington F, White CL, Twigger KR, Rose A, Scott K, Steele L et al. Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. Gene Therapy 2008; 15: 1257–1270.
Kubo H, Ashida A, Matsumoto K, Kageshita T, Yamamoto A, Saida T et al. Interferon-â therapy for malignant melanoma: the dose is crucial for inhibition of proliferation and induction of apoptosis of melanoma cells. Arch Dermatol Res 2008; 300: 297–301.
Kawai T, Akira S . Innate immune recognition of viral infection. Nat Immunol 2006; 7: 131–137.
Pichlmair A, Sousa CRE . Innate Recognition of Viruses. Immunity 2007; 27: 370–383.
Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13: 1050–1059.
Huang B, Sikorski R, Kirn DH, Thorne SH . Synergistic anti-tumor effects between oncolytic vaccinia virus and paclitaxel are mediated by the IFN response and HMGB1. Gene Therapy 2011; 18: 164–172.
Prestwich RJ, Errington F, Steele LP, Ilett EJ, Morgan RS, Harrington KJ et al. Reciprocal human dendritic cell–natural killer cell interactions induce antitumor activity following tumor cell infection by oncolytic reovirus. J Immunol 2009; 183: 4312–4321.
White CL, Twigger KR, Vidal L, De Bono JS, Coffey M, Heinemann L et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Therapy 2008; 15: 911–920.
Ohgimoto K, Ohgimoto S, Ihara T, Mizuta H, Ishido S, Ayata M et al. Difference in production of infectious wild-type measles and vaccine viruses in monocyte-derived dendritic cells. Virus Res 2007; 123: 1–8.
Schneider-Schaulies S, Schneider-Schaulies J . Measles virus-induced immunosuppression. Curr Top Microbiol Immunol 2009; 330: 243–269.
Yang L, Carbone DP . Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res 2004; 92: 13–27.
Steele L, Errington F, Prestwich R, Ilett E, Harrington K, Pandha H et al. Pro-inflammatory cytokine/chemokine production by reovirus treated melanoma cells is PKR/NFkB mediated and supports innate and adaptive anti-tumour immune priming. Mol Cancer 2011; 10: 20.
Galivo F, Diaz RM, Wongthida P, Thompson J, Kottke T, Barber G et al. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Therapy 2009; 17: 158–170.
Liu C, Hasegawa K, Russell SJ, Sadelain M, Peng K-W . Prostate-specific membrane antigen retargeted measles virotherapy for the treatment of prostate cancer. Prostate 2009; 69: 1128–1141.
Msaouel P, Iankov ID, Allen C, Aderca I, Federspiel MJ, Tindall DJ et al. Noninvasive imaging and radiovirotherapy of prostate cancer using an oncolytic measles virus expressing the sodium iodide symporter. Mol Ther 2009; 17: 2041–2048.
Iankov ID, Msaouel P, Allen C, Federspiel MJ, Bulur PA, Dietz AB et al. Demonstration of anti-tumor activity of oncolytic measles virus strains in a malignant pleural effusion breast cancer model. Breast Cancer Res Treat 2010; 122: 745–754.
Myers R, Harvey M, Kaufmann TJ, Greiner SM, Krempski JW, Raffel C et al. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum Gene Ther 2008; 19: 690–698.
Dingli D, Offord C, Myers R, Peng KW, Carr TW, Josic K et al. Dynamics of multiple myeloma tumor therapy with a recombinant measles virus. Cancer Gene Ther 2009; 16: 873–882.
Haralambieva I, Iankov I, Hasegawa K, Harvey M, Russell SJ, Peng KW et al. Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Mol Ther 2007; 15: 588–597.
Li H, Peng K-W, Dingli D, Kratzke RA, Russell SJ . Oncolytic measles viruses encoding interferon beta and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther 2010; 17: 550–558.
Critchley-Thorne RJ, Simons DL, Yan N, Miyahira AK, Dirbas FM, Johnson DL et al. Impaired interferon signaling is a common immune defect in human cancer. Pro Nat Acad Sci 2009; 106: 9010–9015.
Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med 2000; 6: 821–825.
Willmon CL, Saloura V, Fridlender ZG, Wongthida P, Diaz RM, Thompson J et al. Expression of IFN-{beta} enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma. Cancer Res 2009; 69: 7713–7720.
Mocellin S, Pasquali S, Rossi CR, Nitti D . Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst 2010; 102: 493–501.
Iwasaki A, Medzhitov R . Toll-like receptor control of the adaptive immune responses. Nature immunol 2004; 5: 987–995.
Scaffidi P, Misteli T, Bianchi ME . Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002; 418: 191–195.
Bianchi ME, Manfredi AA . High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev 2007; 220: 35–46.
Lotze MT, Tracey KJ . High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 2005; 5: 331–342.
Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A . HMGB1: endogenous danger signaling. Mol Med 2008; 14: 476–484.
Wongthida P, Diaz RM, Galivo F, Kottke T, Thompson J, Pulido J et al. Type III IFN Interleukin-28 mediates the antitumor efficacy of oncolytic virus VSV in immune-competent mouse models of cancer. Cancer Res 2010; 70: 4539–4549.
Banaszynski LA, Sellmyer MA, Contag CH, Wandless TJ, Thorne SH . Chemical control of protein stability and function in living mice. Nat Med 2008; 14: 1123–1127.
Ungerechts G, Springfeld C, Frenzke ME, Lampe J, Parker WB, Sorscher EJ et al. An immunocompetent murine model for oncolysis with an armed and targeted measles virus. Mol Ther 2007; 15: 1991–1997.
Rosenberg SA, Dudley ME . Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol 2009; 21: 233–240.
Duprex WP, McQuaid S, Hangartner L, Billeter MA, Rima BK . Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J Virol 1999; 73: 9568–9575.
Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dötsch C et al. Rescue of measles viruses from cloned DNA. EMBO J 1995; 14: 5773–5784.
Errington F, Jones J, Merrick A, Bateman A, Harrington K, Gough M et al. Fusogenic membrane glycoprotein-mediated tumour cell fusion activates human dendritic cells for enhanced IL-12 production and T-cell priming. Gene Therapy 2006; 13: 138–149.
Acknowledgements
The work described was supported by grants from the Medical Research Council (UK) and Cancer Research UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Stephen J Russell is a named inventor on patents pertaining to the use of MV as an anticancer drug therapy. These patents are owned by Mayo Clinic. The authors otherwise declare no competing interests in relation to the work described.
Rights and permissions
About this article
Cite this article
Donnelly, O., Errington-Mais, F., Steele, L. et al. Measles virus causes immunogenic cell death in human melanoma. Gene Ther 20, 7–15 (2013). https://doi.org/10.1038/gt.2011.205
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.205
Keywords
This article is cited by
-
Mesenchymal stem cell-released oncolytic virus: an innovative strategy for cancer treatment
Cell Communication and Signaling (2023)
-
Oncolytic virotherapy: basic principles, recent advances and future directions
Signal Transduction and Targeted Therapy (2023)
-
Oncolytic attenuated measles virus encoding NY-ESO-1 induces HLA I and II presentation of this tumor antigen by melanoma and dendritic cells
Cancer Immunology, Immunotherapy (2023)
-
Revisiting the melanomagenic pathways and current therapeutic approaches
Molecular Biology Reports (2022)
-
Natural Killer Cells Recruitment in Oncolytic Virotherapy: A Mathematical Model
Bulletin of Mathematical Biology (2021)