Abstract
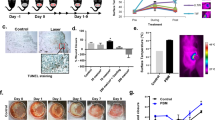
Liposomal gene transfer effectively enhances dermal and epidermal regeneration in burned rodents. To advance this treatment to clinical studies, we investigated the efficacy of liposomal gene transfer in a clinically relevant porcine wound model. Mimicking the clinical scenario, six female Yorkshire pigs (40–50 kg) received up to 12 burns of 50 cm2 area that were fully excised and covered with skin autograft meshed at 4:1 ratio 24 h post-burn. Animals received control injections (empty liposomes), liposomes (DMRIE-C) containing 1 mg LacZ-cDNA, or liposomes (DMRIE-C) with 1 mg of platelet-derived growth factor (PDGF)-cDNA, or the naked PDGF gene. Serial biopsies were taken from different wound sites at multiple time points up to 12 days post-wounding. Transfection efficacy and transfection rate of LacZ and localization of β-gal were determined by immunohistochemical and immunofluorescent techniques. RT-PCR and multiplex protein analysis (ELISA) were used to measure levels of growth factor mRNA transcribed and growth factor protein translated. Wound re-epithelialization and graft adhesion was evaluated using planimetric analysis and clinical scores. We found that peak transfection of liposomal β-galactosidase occurred on day 2, with a fluorescence increase of 154% to baseline (P<0.001). Transfection intensity dropped to 115% above baseline on day 4 (P<0.001) and 109% on day 7. Immunohistochemistry showed a maximum transfection rate of 34% of cells in wound tissue. Gene transfer of liposomal PDGF-cDNA resulted in increased PDGF-mRNA and protein expression on days 2 and 4, and accelerated wound re-epithlialization as well as graft adhesion on day 9 (P<0.05). In this study, we showed that liposomal cDNA gene transfer is possible in a porcine wound model, and by using PDGF-cDNA we further showed that dermal and epidermal regeneration can be improved. These data indicate that liposomal gene transfer can be a new therapeutic approach to improve wound healing in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Branski LK, Pereira CT, Herndon DN, Jeschke MG . Gene therapy in wound healing: present status and future directions. Gene Ther 2007; 14: 1–10.
Singer AJ, Clark RA . Cutaneous wound healing. N Engl J Med 1999; 341: 738–746.
Ammann AJ, Beck LS, DeGuzman L, Hirabayashi SE, Lee WP, McFatridge L et al. Transforming growth factor-beta. Effect on soft tissue repair. Ann NY Acad Sci 1990; 593: 124–134.
Miller CR, Bondurant B, McLean SD, McGovern KA, O'Brien DF . Liposome-cell interactions in vitro: effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry 1998; 37: 12875–12883.
Branski LK, Mittermayr R, Herndon DN, Norbury WB, Masters OE, Hofmann M et al. A porcine model of full-thickness burn, excision and skin autografting. Burns 2008; 34: 1119–1127.
Meyer W, Schwarz R, Neurand K . The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr Probl Dermatol 1978; 7: 39–52.
Forbes PD . Vascular supply of the skin and hair in swine. In: Montagna W, Dobson RL (eds). Advances in the Biology of Skin: Hair Growth, vol. 9. Pergamon: Oxford, 1969, pp 419–432.
Heinrich W, Lange PM, Stirtz T, Iancu C, Heidemann E . Isolation and characterization of the large cyanogen bromide peptides from the alpha1- and alpha2-chains of pig skin collagen. FEBS Lett 1971; 16: 63–67.
Winter GD . A Study of Wound Healing in the Domestic Pig. PhD thesis, Birbeck College, University of London: London, 1966.
Gray GM, White RJ, Williams RH, Yardley HJ . Lipid composition of the superficial stratum corneum cells of pig epidermis. Br J Dermatol 1982; 106: 59–63.
Sullivan TP, Eaglstein WH, Davis SC, Mertz P . The pig as a model for human wound healing. Wound Repair Regen 2001; 9: 66–76.
Mast BA, Schultz GS . Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Rep Reg 1996; 4: 411–420.
Steed DL . Modifying the wound healing response with exogenous growth factors. Clin Plast Surg 1998; 25: 397–405.
Robson MC, Smith PD . Topical use of growth factors to enhance healing. In: Falanga V (ed). Cutaneous Wound Healing. Taylor & Francis: London, 2001, pp 379–398.
Lynch SE, Nixon JC, Colvin RB, Antoniades HN . Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci USA 1987; 84: 7696–7700.
Noguchi A, Furuno T, Kawaura C, Nakanishi M . Membrane fusion plays an important role in gene transfection mediated by cationic liposomes. FEBS Lett 1998; 433: 169–173.
Williams RS, Johnston SA, Riedy M, DeVit MJ, McElligott SG, Sanford JC . Introduction of foreign genes into tissues of living mice by DNA-coated microprojectiles. Proc Natl Acad Sci USA 1991; 88: 2726–2730.
Felgner PL, Ringold GM . Cationic liposome-mediated transfection. Nature 1989; 337: 387–388.
Jeschke MG, Barrow RE, Hawkins HK, Tao Z, Perez-Polo JR, Herndon DN . Biodistribution and feasibility of non-viral IGF-I gene transfers in thermally injured skin. Lab Invest 2000; 80: 151–158.
Eming SA, Whitsitt JS, He L, Krieg T, Morgan JR, Davidson JM . Particle-mediated gene transfer of PDGF isoforms promotes wound repair. J Invest Dermatol 1999; 112: 297–302.
Jeschke MG, Barrow RE, Hawkins HK, Yang K, Hayes RL, Lichtenbelt BJ et al. IGF-I gene transfer in thermally injured rats. Gene Ther 1999; 6: 1015–1020.
Sun L, Xu L, Chang H, Henry FA, Miller RM, Harmon JM et al. Transfection with aFGF cDNA improves wound healing. J Invest Dermatol 1997; 108: 313–318.
Jeschke MG, Schubert T, Klein D . Exogenous liposomal IGF-I cDNA gene transfer leads to endogenous cellular and physiological responses in an acute wound. Am J Physiol Regul Integr Comp Physiol 2004; 286: R958–R966.
Jeschke MG, Barrow RE, Hawkins HK, Chrysopoulo MT, Perez-Polo JR, Herndon DN . Effect of multiple gene transfers of insulin like growth factor I complementary DNA gene constructs in rats after thermal injury. Arch Surg 1999; 134: 1137–1141.
Jeschke MG, Klein D . Liposomal gene transfer of multiple genes is more effective than gene transfer of a single gene. Gene Ther 2004; 11: 847–855.
Ross R, Glomset J, Kariya B, Harker L . A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci USA 1974; 71: 1207–1210.
Senior RM, Griffin GL, Huang JS, Walz DA, Deuel TF . Chemotactic activity of platelet alpha granule proteins for fibroblasts. J Cell Biol 1983; 96: 382–385.
Tzeng DY, Deuel TF, Huang JS, Baehner RL . Platelet-derived growth factor promotes human peripheral monocyte activation. Blood 1985; 66: 179–183.
Tzeng DY, Deuel TF, Huang JS, Senior RM, Boxer LA, Baehner RL . Platelet-derived growth factor promotes polymorphonuclear leukocyte activation. Blood 1984; 64: 1123–1128.
Ansel JC, Tiesman JP, Olerud JE, Krueger JG, Krane JF, Tara DC et al. Human keratinocytes are a major source of cutaneous platelet-derived growth factor. J Clin Invest 1993; 92: 671–678.
Liu PY, Liu K, Wang XT, Badiavas E, Rieger-Christ KM, Tang JB et al. Efficacy of combination gene therapy with multiple growth factor cDNAs to enhance skin flap survival in a rat model. DNA Cell Biol 2005; 24: 751–757.
Wang XT, Liu PY, Tang JB . PDGF gene therapy enhances expression of VEGF and bFGF genes and activates the NF-kappaB gene in signal pathways in ischemic flaps. Plast Reconstr Surg 2006; 117: 129–139.
Keswani SG, Katz AB, Lim FY, Zoltick P, Radu A, Alaee D et al. Adenoviral mediated gene transfer of PDGF-B enhances wound healing in type I and type II diabetic wounds. Wound Repair Regen 2004; 12: 497–504.
Gu DL, Nguyen T, Gonzalez AM, Printz MA, Pierce GF, Sosnowski BA et al. Adenovirus encoding human platelet-derived growth factor-B delivered in collagen exhibits safety, biodistribution, and immunogenicity profiles favorable for clinical use. Mol Ther 2004; 9: 699–711.
Furst W, Banerjee A, Redl H . Comparison of structure, strength and cytocompatibility of a fibrin matrix supplemented either with tranexamic acid or aprotinin. J Biomed Mater Res B Appl Biomater 2006; 82: 109–114.
Foss DL, Baarsch MJ, Murtaugh MP . Regulation of hypoxanthine phosphoribosyltransferase, glyceraldehyde-3-phosphate dehydrogenase and beta-actin mRNA expression in porcine immune cells and tissues. Animal Biotechnol 1998; 9: 67–78.
Acknowledgements
We acknowledge the entire staff of the large animal facility of the Shriners Hospitals for Children, especially Thomas Miszalkiewski and John R Salsbury, for their great support in the conduction of the animal studies. We also want to thank Zafar Khakpour, LBI Vienna, for the design and construction of the burn apparatus, and Pat Sellers of the Pathology core laboratory for the preparation of histological slides. We thank Baxter AG, Vienna, Austria, and especially Dr Melitta Bilban, for supporting this study. The work was supported by a Shriners Hospitals for Children (SHC) Research Fellowship Grant no. 8505 and SHC Project/Facility Grants no. 8450, 8460 and 8480. Further support was granted by the Clayton Foundation of Houston.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Branski, L., Masters, O., Herndon, D. et al. Pre-clinical evaluation of liposomal gene transfer to improve dermal and epidermal regeneration. Gene Ther 17, 770–778 (2010). https://doi.org/10.1038/gt.2010.32
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2010.32
Keywords
This article is cited by
-
Functional characterization of the dimeric form of PDGF-derived fusion peptide fabricated based on theoretical arguments
Scientific Reports (2024)
-
Basic concepts, current evidence, and future potential for gene therapy in managing cutaneous wounds
Biotechnology Letters (2019)