Abstract
Background:
Probiotic functional foods are widely advertised to consumers primarily based on probiotic supplements.
Objective:
Determine if consumption of yogurt containing a high dose of probiotics improves health in children ages 1–3 years attending daycare/school centers.
Subjects/Methods:
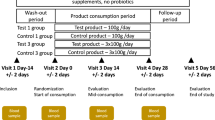
Double-blinded, randomized, placebo-controlled, allocation concealment clinical trial. Setting: Outpatient participants in the Washington, DC area. Participants: 182 healthy children between the age of 1 and 3 years attending daycare/school at least 3 days a week. Intervention: Active was a strawberry yogurt-based drink supplemented with Bifidobacterium animalis ssp. lactis (B. lactis) BB-12. The placebo was indistinguishable from the active drink, differing only in absence of the probiotic BB-12. Primary objective was to determine if consumption of a probiotic-containing yogurt-based drink decreases absences due to illnesses from daycare for children ages 1–3 years. Secondary was to determine if probiotic-containing yogurt-based drink improves overall parental satisfaction due to decreased absences from work and an overall healthier child.
Results:
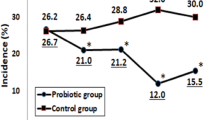
There were no significant differences in the days of missed school per group, with 51.9% in the active group and 47.1% in the placebo group missing at least 1 day of school throughout the study. Additionally, there were no differences in any secondary outcomes among the groups.
Conclusions:
Consumption of a yogurt-based drink delivering 1010 CFU of Bifidobacterium animalis ssp. lactis (B. lactis) BB-12 per day did not decrease the number of days missed of school due to an illness. Additional independent research on the potential of BB-12 to reduce illness in children needs to be conducted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Abbreviations
- BB-12:
-
Bifidobacterium animalis ssp. lactis (B. lactis)
- AE:
-
adverse events
- SAE:
-
serious adverse events
References
Alfaleh K, Bassler D (2008). Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev CD005496.
Bartosch S, Woodmansey EJ, Paterson JC, McMurdo ME, Macfarlane GT (2005). Microbiological effects of consuming a synbiotic containing Bifidobacterium bifidum, Bifidobacterium lactis, and oligofructose in elderly persons, determined by real-time polymerase chain reaction and counting of viable bacteria. Clin Infect Dis 40, 28–37.
Fleming DW, Cochi SL, Hightower AW, Broome CV (1987). Childhood upper respiratory tract infections: to what degree is incidence affected by day-care attendance? Pediatrics 79, 55–60.
Food and Agriculture Organization of the United Nations and World Health Organization (2001). Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Córdoba, Argentina, 1–4 October 2001.
Fukushima Y, Li ST, Hara H, Terada A, Mitsuoka T (1997). Effect of follow-up formula containing Bifidobacteria (NAN BF) on fecal flora and fecal metabolites in healthy children. Biosci Microflora 16, 65–72.
Fukushima Y, Kawata Y, Hara H, Terada A, Mitsuoka T (1998). Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int J Food Microbiol 42, 39–44.
Haskins R (1989). Acute illness in day care: how much does it cost? Bull NY Acad Med 65, 319–343.
Hatakka K, Savilahti E, Ponka A, Meurman JH, Poussa T, Nase L et al. (2001). Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ 322, 1327.
Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S (2000). Probiotics in the management of atopic eczema. Clin Exp Allergy 30, 1604–1610.
Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E (2001). Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357, 1076–1079.
Leyer GJ, Li S, Mubasher ME, Reifer C, Ouwehand AC (2009). Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics 124, e172–e179.
Lin JS, Chiu YH, Lin NT, Chu CH, Huang KC, Liao KW et al. (2009). Different effects of probiotic species/strains on infections in preschool children: a double-blind, randomized, controlled study. Vaccine 27, 1073–1079.
Malinen E, Matto J, Salmitie M, Alander M, Saarela M, Palva A (2002). PCR-ELISA II: analysis of Bifidobacterium populations in human faecal samples from a consumption trial with Bifidobacterium lactis Bb-12 and a galacto-oligosaccharide preparation. Syst Appl Microbiol 25, 249–258.
Merenstein DJ, Foster J, D’Amico F (2009). A randomized clinical trial measuring the influence of kefir on antibiotic-associated diarrhea: the measuring the influence of Kefir (MILK) Study. Arch Pediatr Adolesc Med 163, 750–754.
Ouwehand AC, Kurvinen T, Rissanen P (2004). Use of a probiotic Bifidobacterium in a dry food matrix, an in vivo study. Int J Food Microbiol 95, 103–106.
Pedone CA, Bernabeu AO, Postaire ER, Bouley CF, Reinert P (1999). The effect of supplementation with milk fermented by Lactobacillus casei (strain DN-114 001) on acute diarrhoea in children attending day care centres. Int J Clin Pract 53, 179–184.
Rosenfeldt V, Michaelsen KF, Jakobsen M, Larsen CN, Moller PL, Tvede M et al. (2002). Effect of probiotic Lactobacillus strains on acute diarrhea in a cohort of nonhospitalized children attending day-care centers. Pediatr Infect Dis J 21, 417–419.
Shonkoff JP (2003). From neurons to neighborhoods: old and new challenges for developmental and behavioral pediatrics. J Dev Behav Pediatr 24, 70–76.
Silverstein M, Sales AE, Koepsell TD (2003). Health care utilization and expenditures associated with child care attendance: a nationally representative sample. Pediatrics 111, e371–e375.
StataCorp (2005). Stata Statistical Software: Release 9. StataCorp LP: College Station, TX.
Vanderhoof J, Whitney D, Antonson D (2000). In children receiving antibiotics, does coadministration of lactobacillus GG reduce the incidence of diarrhea? West J Med 173, 397.
Ventura M, Reniero R, Zink R (2001). Specific identification and targeted characterization of Bifidobacterium lactis from different environmental isolates by a combined multiplex-PCR approach. Applied and Environmental Microbiology 67, 2760–2765.
Weizman Z, Asli G, Alsheikh A (2005). Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics 115, 5–9.
Acknowledgements
We would like to thank Jaclyn N. McCallister, research assistant, CAPRICORN research coordinators Haewon Park and Tina Tan, for their invaluable help, without whom this study would not have occurred. Dr's Mirjana Curic and Dave McCoy from Chr. Hansen for their help in obtaining cultures and help throughout the study. We would also like to thank the Data Safety Monitoring Board, Dr Seiji Hayashi George Washington University; Dr David Meyers the Agency for Healthcare Research and Quality and Dr Susan Crystal-Mansour community representative. We are also indebted to Cathy Obits Program Manager of the Gerber Foundation and the entire Foundation for their help and financial support.
Study concept and design: Merenstein, Roberts, Petterson, Sanders.
Acquisition of data: Merenstein, Scriven, Herbin-Smith.
Analysis and interpretation of data: Merenstein, Scriven, Herbin-Smith, Roberts, Petterson, Sanders.
Initial Drafting of the manuscript: Merenstein, Scriven, Herbin-Smith.
Critical revision of the manuscript for important intellectual content: Roberts, Petterson, Sander.
Statistical analysis: Petterson, Merenstein.
Obtained funding: Merenstein.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Mary Ellen Sanders consults for numerous different probiotic manufacturers. No other authors report conflict of interest.
Additional information
Contributors: This study was funded by The Gerber Foundation. The Gerber Foundation supports research regarding the health and nutrition of infants and young children from 0 to 3 years of age. PI had full legal ability to publish any findings.
Rights and permissions
About this article
Cite this article
Merenstein, D., Smith, K., Scriven, M. et al. The study to investigate the potential benefits of probiotics in yogurt, a patient-oriented, double-blind, cluster-randomised, placebo-controlled, clinical trial. Eur J Clin Nutr 64, 685–691 (2010). https://doi.org/10.1038/ejcn.2010.30
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2010.30
Keywords
This article is cited by
-
The relationship between dairy products intake and breast cancer incidence: a meta-analysis of observational studies
BMC Cancer (2021)
-
Probiotics for respiratory tract infections in children attending day care centers—a systematic review
European Journal of Pediatrics (2018)