Abstract
The 26S proteasome is a large, ∼2.5 MDa, multi-catalytic ATP-dependent protease complex that serves as the degrading arm of the ubiquitin system, which is the major pathway for regulated degradation of cytosolic, nuclear and membrane proteins in all eukaryotic organisms.
Similar content being viewed by others
Main
As proteasome-mediated degradation regulates the turnover of numerous cellular proteins involved in essentially all cellular processes, its own regulation plays key roles in preserving homeostasis. Proteasome activity is regulated at several levels, ranging from its abundance, i.e., the synthesis of its subunits; the rate of its assembly and disassembly; its regulation by post translational modifications (PTMs); regulation of the events related to its proteolytic activity, i.e., substrate recognition and binding, subsequent conformational changes of the proteasome, substrate deubiquitination, unfolding, and translocation into the catalytic chamber; proteasome subcellular localization and its recruitment to specific organelles, and finally, the destruction of the proteasome itself accomplished either via degradation of individual subunits or by removal of the proteasome as a whole.
All the above events are responsive to the changing cellular environment and different pathophysiological conditions. In this review, we discuss the current knowledge regarding the regulatory processes that underlie the basis for proper proteasome function and its adjustment to the changing requirements of the cell.
Note that nomenclature of proteasomal subunits and effectors differs between organisms. Referring to proteasome subunits, we adopt the yeast protein terms: α, β, Rpn and Rpt subunits. Effector ortholog names are introduced once, and are later referred to using one representative name.
The 26S proteasome structure and function
The 26S proteasome consists of two distinct sub-complexes, a 20S core particle (CP) and a 19S regulatory particle (RP, also termed PA700). The 20S CP is composed of four axially stacked heteroheptameric rings (two outer α- and two inner β-rings), and has a barrel-shaped structure1 (Figure 1). The outer α-rings contain seven similar, yet distinct α-subunits (α1-α7), and by forming a pore, they function as a tightly regulated “gate” for the entrance of substrates, and for removal of degradation products from the complex. This “gate” which is made of the N-termini of a subset of α-subunits, blocks the unregulated entrance of substrates into the catalytic chamber. A crucial role in the organization and activation of the “gate” is attributed to the N-terminus of the α3-subunit, since its deletion results in a constitutively open pore2. The mechanism of the “gate” opening and proteasome activity are regulated by the docking of proteasome regulators (such as 19S RP, PA28, PA200, ECM29 and PI31) containing an HbYX motif (where Hb stands for a hydrophobic residue; Y for tyrosine; and X for any amino acid) onto seven binding pockets formed by α-α interfaces on the 19S-facing surface of the outer α-rings3,4. In addition, the outer α-rings form extra interior compartments, the “antechambers”, which are connected to the central chamber of the “barrel” from each side, and can keep a certain amount of intact substrate or digested products5,6. Also, several of the α-subunits have an important role in the subcellular localization of the proteasome by bearing a nuclear localization signal (NLS)7,8,9.
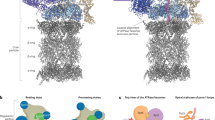
The life cycle of the proteasome. The “birth” of the proteasome is controlled by transcriptional regulation of its different subunits. The biogenesis is organized by transcription factors such as Nrf1 and Rpn4, which are sensitive to changing physiological conditions. The different subunits assemble in a coordinated manner to form the mature proteasome. The 26S proteasome recognizes ubiquitin conjugated substrates in a process mediated by intrinsic and extrinsic ubiquitin receptors. The recognition is regulated by different post-translational modifi cations, disassembly, conformational changes, and cellular localization that the proteasome undergoes. The “death” of the proteasome is at least partially mediated by the lysosome/vacuole/autophagy and cleavage by caspase(s). In the middle is the energy-dependent ubiquitin-substrate conjugate formation catalyzed by E1, E2, and E3.
Similarly, the inner β-rings consist of seven distinct β-subunits (β1-β7), which are flanked by the two outer α-rings. Three of the β-subunits, β1, β2 and β5, contain active sites with different proteolytic specificities: the peptidyl-glutamyl-hydrolyzing or caspase-like, the trypsin-like, and the chymotrypsin-like activity, respectively. The catalytic β-subunits are synthesized as precursors bearing N-terminal propeptides. The elimination of the propeptides during proteasomal maturation is required for exposure of the N-terminal catalytic threonine (Thr) residue. Hence, each mature eukaryotic proteasome has six proteolytic sites with three types of proteolytic activities6,10.
Additional “specializing” β-subunits have been identified in mammalian cells under specific conditions/organs: β1i, 2i, β5i and β5t, where “i” stands for immunoproteasome and “t” for thymoproteasome.
The thymoproteasome was found only in cortical epithelial cells of the thymus and is thought to play a vital role in the positive selection of CD8+ T-cells. The configuration of the active site of the thymoproteasome is β1i-β2i-β5t and their chymotrypsin-like activity is lower in comparison with “standard” and immunoproteasomes11,12.
Immuno-β-subunits are commonly expressed in a broad variety of immune system-specific tissues like the spleen, thymus, lung, liver, kidney, colon, small intestine and antigen-presenting cells (APCs). Their expression can also be induced in non-immune tissues (or cells) by specific (e.g., IFN-γ, TNF-α, LPS) and less specific (e.g., aging and environmental stress factors) stimuli11. The proteolytic activity of the immunoproteasome has an altered specificity toward cleavage after basic and hydrophobic residues that are thought to increase the affinity of the substrate fragments to MHC class I molecules13. Analysis of proteasomes from different tissues shows that a single 26S holoenzyme can be made of both “constitutive” and “immunological” β-subunits, thus generating an intermediate (or hybrid) proteasome subpopulation11.
The “canonical” proteasome cap, the 19S RP, is a multifunctional complex which regulates proteasome function by identification, binding, deubiquitination, unfolding and translocation of substrates to the proteolytic chamber of the CP. The RP is further divided into two additional subcomplexes, the “base” and “lid”. The base consists of six regulatory particle AAA ATPase subunits (Rpt1-Rpt6), organized into a ring, as well as four regulatory particle non-ATPase subunits (Rpn1, Rpn2, Rpn10 and Rpn13 (Adrm1))12. Rpn1, Rpn10 and Rpn13 serve as ubiquitin receptors, recognizing substrates targeted to the proteasome14,15,16. The lid consists of nine different Rpn subunits (Rpn3, Rpn5-9, Rpn11, Rpn12 and Rpn15 (Dss1/Sem1)), which form a horseshoe-shaped structure. A main function of the lid is deubiquitination of incoming substrates. This activity is carried out by the deubiquitinating enzymes (DUBs) Rpn11, Uch37 and Ubp6/Usp1417,18,19,20,21. Interestingly, high-energy nucleotides are required in order to hold the 19S and 20S sub-complexes together and for opening the “gate” to the catalytic chamber by coordinating the timed separation and proper movement of the α-ring N-termini22,23. Certain important functions of the 19S RP are energy-dependent; among them is “preparation” of the substrates and their translocation into the CP for degradation1,24. The various functions of the 19S RP are described below, and are reviewed in details in a more biochemically based review25.
Assembly of the 26S proteasome
The assembly of the proteasome is a highly complex, multi-step process, accompanied by proteasome-dedicated chaperones and maturation factors. The positioning of each individual subunit in the final structure of the mature proteasome is highly defined.
Assembly of the eukaryotic 20S proteasome is initiated by the formation of an α-ring, which is controlled and directed by two main heterodimeric chaperone complexes: proteasome-assembling chaperone 1 (PAC1)•PAC2 and PAC3•PAC4. One model for α-ring formation suggests that it is initiated by the interaction of the α5 and α7 subunits with the PAC1•PAC2 complex. The complex then mediates the incorporation of the rest of the α subunits, and prevents spontaneous dimerization of either α subunits or complete α-rings26. The PAC1•PAC2 complex stays bound to the outer side of the α-ring until the complete assembly of the 20S proteasome, and is thought to protect it from premature docking of activators27. The PAC3•PAC4 complex is also involved in early steps of the α-ring formation by acting along with PAC1•PAC2, preventing the incorrect incorporation of α-subunits during α-ring formation28. The PAC3•PAC4 complex is bound to the inner side of the α-ring. The complete formation of the heptameric α-ring initiates the assembly of the half-proteasome, consisting of one α- and one β-ring. In mammals, the α-ring serves as an assembly platform for the β-ring, the formation of which starts with β2, followed by β3, β4, β5, β6 and β1 subunits. The formation of the β-ring is initiated by incorporation of UMP1, another chaperone, into the α-ring prior or concomitant with β2. The incorporation of the β3 subunit triggers the release of the PAC3•PAC4 complex. One α-ring with incorporated β2, β3, and β4 subunits, forms an intermediate structure, termed the 13S complex29. UMP1 regulates the correct incorporation order of the other β subunits. In addition, UMP1 localizes the immature proteasome to the endoplasmic reticulum (ER), the main assembly site of the proteasome in mammalian cells30. Most of the β-subunits, excluding β3 and β4, are synthesized as precursors with N-terminal propeptides. The propeptides of β2 and β5 are essential for recruitment and incorporation of β3 and β6, respectively. The β5 propeptide is also necessary for specific interaction with UMP131. The propeptides of β1, β2 and β5 prevent the premature activity of their N-terminal catalytic Thr residue. Furthermore, the C-terminal tails of β-subunits have an important role in proteasome biogenesis by providing a specific interaction within or between β-rings. The β-ring formation is terminated by the incorporation of β7, hence forming a half 20S proteasome, called the 15S complex (α1-7β1-7-UMP1-PAC1•PAC2). The incorporation of β7 induces the dimerization of two half-mers by insertion of its C-terminal tail into a groove between β1 and β2 in the opposite β-ring. Upon dimerization, the propeptides of the β-subunits undergo autocatalytic cleavage, exposing their catalytic Thr residue24,32. Finally, the PAC1•PAC2 complex and UMP1 are degraded by the mature 20S proteasome24,26,31.
By contrast, the assembly of the 19S proteasome is still not a well understood process. The lid and base sub-complexes of the 19S are assembled independently, which precedes their association with one another through Rpn10. The base formation is assisted by a group of base-dedicated chaperones, arranging the six ATPase subunits in a defined order in a ring, and also inhibiting premature DUBs and ATPase catalytic activities1. The base-dedicated chaperones expressed in yeast are Hsm3, Nas2, Nas6 and Rpn14, and their mammalian homologs are S5b, p27, gankyrin/p28 and Rpn14/PAAF1 (proteasomal ATPase-associated factor 1), respectively. In addition, Adc17 is a stress-induced chaperone found in yeast, which was shown to facilitate 19S RP assembly in response to changing demands33. These chaperones bind through their C-terminal protein-protein interacting domain to specific ATPase subunits1,32. Base assembly starts with the formation of three Rpt cis-trans heterodimers (Rpt1:Rpt2, Rpt4:Rpt5, Rpt3:Rpt6), associated with specific chaperones (Hsm3/S5b-Rpt1:Rpt2-Rpn1, Rpt4:Rpt5-Nas2/p27, Nas6/gankyrin/p28-Rpt3:Rpt6-Rpn14/PAAF1), which are involved in the pairing process. Adc17 was also shown to support Rpt3:Rpt6 pairing33. The exact order of assembly of the different pairs to the final base structure is unclear, yet the process is accomplished by subsequent joining of all pairs, as well as that of Rpn2 and Rpn13 subunits34.
The lid assembly is a much less understood process. However, it has been suggested that it also occurs in several steps. The assembly starts with formation of two modules: a core module, consisting of Rpn5-6, Rpn8-9 and Rpn11, and a second module, consisting of Rpn3, Rpn7 and Rpn15/Sem1. The joining of the two modules is mediated by interaction between Rpn3 and Rpn5. The incorporation of Rpn12 completes the lid formation1. In addition, a recent study showed that incorporation of the Rpn12 subunit triggers a conformational change in the forming lid (mediated by a single helix of Rpn12), which results in its association of the base35. No lid-specific assembly chaperones have been discovered yet. However, it has been suggested that Hsp90 contributes to its assembly in yeast36.
Proteasomal regulation
Transcriptional regulation of proteasome biogenesis
Current knowledge regarding the basal rate of proteasome subunit biosynthesis is limited. It is believed that all subunits of the “canonical” proteasome are found in cells only in the context of their respective complex, the 20S or 19S, with the exception of the 19S subunit Rpn10/S5a, which is also present in a free state37, though not necessarily in all cell types38. Since proteasomal subunits seem to incorporate into their sub-complexes with unassembled subunits being removed39, it is difficult to determine whether the stoichiometry observed in intact complexes faithfully represents the rate of individual subunit's synthesis. Nevertheless, recent studies demonstrated the concerted, yet not necessarily even, upregulation of proteasome subunits in response to stress40,41,42, contributing to the growing body of evidence for common signaling pathways regulating proteasome gene expression.
Upon proteasome inhibition, a concerted de novo synthesis of all 26S proteasomal subunits with subsequent whole proteasome formation was observed in several organisms40,43,44,45,46. In mammals, it has been shown that nuclear factor erythroid 2-related factor 1 (Nrf1) is a transcription factor (TF) essential for the activation of proteasomal gene expression in response to proteasome inhibition40,47,48. Nrf1 was reported to be ubiquitinated by more than one E3 ubiquitin ligase (HRD1, Fbw7/FBXW7, and β-TrCP), and is possibly degraded by the proteasome42,47,48. Normally, Nrf1 is an ER-bound protein. Its release and translocation to the nucleus to activate transcription requires its deglycosylation, ubiquitination, and partial proteasomal degradation (i.e., processing). Interestingly, the addition of a low concentration of a proteasome inhibitor results in processing and nuclear localization of Nrf1, upregulation of proteasomal subunits, as well as of other proteasome-related genes, while such response does not occur in the presence of a high concentration of the inhibitor. In the absence of an inhibitor, Nrf1 is rapidly degraded42.
Nrf1, and its homolog Nrf2, are basic leucine zipper TFs, known to bind antioxidant response elements (AREs) in promoters of target genes. Studies showed that many of the proteasomal subunit genes harbor putative AREs in their promoters, so that Nrf1-mediated proteasome upregulation following inhibition of the proteasome may rely on Nrf1 binding to these AREs47,48. Nrf2 is also a substrate of the ubiquitin-proteasome system (UPS). Under normal conditions, it is bound to Keap1 which serves as the substrate-recognizing component of an SCF E3 ubiquitin ligase along with Cul3-Rbx1. The ligase ubiquitinates Nrf2, targeting it for proteasomal degradation. One mechanism suggested to underlie, at least in part, Nrf2 upregulation under oxidative stress is its dissociation from Keap1 in the presence of high concentration of antioxidants, which results in its stabilization and subsequent translocation to the nucleus, where it induces proteasomal genes transcription49,50,51.
In C. elegans, the compensatory proteasome upregulation in response to both proteasome inhibition and oxidative stress was demonstrated to depend on a single TF, SKN-1, which is an ortholog of Nrf1 and Nrf245,52. Expectedly, SKN-1 is also a proteasomal substrate, targeted for degradation following ubiquitination by the CUL4/DDB1 E3 ubiquitin ligase53.
Rpn4 is a short-lived protein that acts as a TF in Saccharomyces cerevisiae. Being a proteasomal substrate, ubiquitinated by the Ubr2 E3 ubiquitin ligase54 or degraded in a ubiquitin-independent manner55, Rpn4 levels are reciprocally correlated with proteasome activity. In face of compromised proteasomal function, Rpn4 level is elevated and its activity as a TF upregulated56. As it enters the nucleus, Rpn4 binds a unique sequence upstream to proteasomal genes, the proteasome-associated control element (PACE), thereby stimulating the expression of proteasomal genes57. As for transcriptional regulation of Rpn4 itself, it was found that many stress-induced TFs harbor recognition motifs upstream to the RPN4 gene; among them are factors related to oxidative stress (YAP1), drug resistance (PDRs), and heat shock (HSF1). These findings suggest a possible role for proteasome upregulation under different stress conditions. Rpn4 was also found to bind upstream to the oxidative stress effector YAP1, further establishing the bilateral linkage between oxidative stress and proteasome level and function58.
Besides common regulatory pathways affecting subunit abundance which respond to alterations in proteasome function or to general stress, an intriguing study suggested that overexpression of a single proteasome subunit, β5, may upregulate the level of other subunits, as well as proteasome assembly and activity. This results in improved cellular function as reflected by ameliorated response to oxidative stress and delayed senescence59.
Another study showed that the upregulation of a single 19S subunit, Rpn6, also stimulates proteasomal activity in C. elegans, though not via elevation of other proteasomal subunits gene expression60. The same group also showed that human embryonic stem cells (hESCs) exhibit higher levels of proteasome activity, an elevation which disappears upon their differentiation in correlation with reduction in the level of Rpn6, with no change in the level of any other regulatory proteasomal subunit. Indeed, lowering Rpn6 level in hESCs resulted in reduced proteasomal activity. Induction of pluripotency (i.e., cell reprogramming into induced pluripotent stem cells, or iPSCs) led to increased proteasomal activity as well as to elevated Rpn6 level. It is suggested that Rpn6 stimulates proteasome activity by increasing the association between the 20S CP and the 19S RP. Accordingly, overexpression of Rpn6 resulted in increased proteasome assembly and activity, with a similar reciprocal effect observed following Rpn6 knockdown61. In both C. elegans and human, the regulation of proteasome assembly and activity through Rpn6 is mediated by the DAF-16/FOXO4 orthologous TFs60,61.
Proteasome activity in response to stress
A recent debate concerning the effect of mechanistic target of rapamycin (mTOR) on the proteasome is highlighting one of the signaling pathways regulating its activity. mTOR is a central regulator of cell growth and proliferation, affecting a broad array of activities, including protein synthesis and breakdown62. One study suggested that activation of mTOR and subsequent increase in protein synthesis, also results in Nrf1-mediated upregulation of proteasome biogenesis and activity63. On the other hand, a later study claimed that inhibition of mTOR, known to induce autophagy, results also in activation of proteasomal degradation, and that this upregulation is independent of de novo protein synthesis64. It is yet unclear whether these two apparently contradicting findings reflect differences in experimental approach and/or pathophysiological conditions of the cells65,66. Nevertheless, it is clear that mTOR downstream effect on proteasome activity plays an important role in its regulation and more studies are required to define the different conditions and direction of the regulatory process.
Proteasome regulation by post-translational modifications
As a tightly regulated enzyme comprised of dozens of subunits, the proteasome is a target for many PTMs, having their number growing steadily.
In a recent meta-analysis, researchers generated a comprehensive map of proteasomal co- and post-translational modifications, detected via employment of proteomic analyses. More than 345 modifications, belonging to 11 different types, were shown to “decorate” the 26S proteasome in yeast67. It has also been shown that the same proteasomal modification site may serve as a target for more than one modification, suggesting a cross talk between different types of modification68. Such data demonstrate the complexity of studying proteasomal regulation by PTMs, while emphasizing the probable magnitude of the phenomenon.
Importantly, the mechanism by which the different modifications exert their effect on proteasomal function is largely unknown. For many of the modifications, even their effect on the proteasome and/or the site/subunit modified have not been deciphered67. Such are the cases, for example, for proteasome dephosphorylation by Ublcp1 (which regulates nuclear proteasome activity69), N-terminal acetylation of proteasomal subunits by N-acetyl transferases (NATs) in yeast (which mediates proteasome localization during aging70 (and for which even the subunit(s) that is modified at its N-terminus has not been identified)), and ubiquitination of the proteasome (targeting it as a substrate for lysosomal degradation71).
Nevertheless, established cases of proteasomal PTMs and their role in proteasome regulation are already at hand. Table 1 lists different PTM types, of which both the target and the effect on proteasomal function are known. As some studies identified only the complex rather than the subunit that is modified, in these cases the table refers to that complex. This may explain an opposite effect (upregulation and downregulation) of a given modification occurring on different subunits.
Substrate recognition and degradation
Ubiquitin receptors
Ubiquitinated substrates are recognized by ubiquitin receptors and targeted to the 26S proteasome for degradation. Ubiquitin receptors can be classified according to their association with the proteasome: the proteasome intrinsic receptors which are subunits of the 19S RP, and the extra-proteasomal proteins that bind ubiquitinated substrates as free entities and shuttle them to the 26S proteasome.
Proteasomal ubiquitin receptors
Three intrinsic proteasomal subunits have been shown to bind ubiquitinated substrates: Rpn1314, Rpn115 and Rpn1016. The 19S subunits Rpt589 and Rpn1590 were also suggested as possible ubiquitin receptors, as they were shown to bind ubiquitin, but whether they actually recognize ubiquitinated substrates targeted to the proteasome is yet to be determined.
Rpn13 binds ubiquitinated substrates via its N-terminal pleckstrin-like receptor of ubiquitin (PRU) domain91, while its C-terminal domain is known to bind and activate the DUB Uch37. Together, they function as a “proofreading” or “editing” machinery that enables the escape of poorly or inadvertently ubiquitinated substrates by removing their ubiquitin moieties92,93, or potentially by trimming the chain to a length that fits the proteasome better, directing the substrate for efficient degradation.
Besides its established role as a receptor for ubiquitinated substrates, Rpn1 has also been shown to mediate proteasomal interaction with several UPS components. Studies in yeast have shown that Rpn1 can serve as a binding site for the shuttle proteins radiation sensitivity abnormal 23 (Rad23), dual-specificity protein kinase 2 (Dsk2)94,95 and DNA damage-inducible 1 (Ddi1)96. Recent study characterized the binding sites of Rpn1 with different interactors, and found that its binding to both ubiquitin and Rad23 UBL domain occurs via the same binding site, T1, and that an adjacent binding site, T2, recognizes the ubiquitin-like (UBL) domain of the extra-proteasomal DUB Ubp615.
Rpn10 is a unique ubiquitin receptor, as it functions both in its proteasome-bound form, as well as in its free state, as shown in D. melanogaster97, S. cerevisiae98, and A. thaliana99. Rpn10 binds polyubiquitin chains via its ubiquitin-interacting motif (UIM)16,97 and harbors a von Willebrand A (VWA) N-terminal domain, which facilitates its binding to the proteasome100 and the subsequent degradation of several ubiquitinated substrates16,101. Monoubiquitination of Rpn10 regulates its capacity to bind ubiquitinated substrates, as it promotes intramolecular interactions that hinder the UIM, reducing its affinity toward ubiquitinated substrates82,102,103.
Non-proteasomal ubiquitin receptors and shuttle proteins
In addition to stoichiometric proteasomal ubiquitin receptors, a group of non-proteasomal UBL-UBA (ubiquitin-associated) shuttle proteins also serves as ubiquitin receptors. Three of these receptors, Rad23, Dsk2 and Ddi1, have been characterized in detail in yeast. These UBL-UBA domain-containing proteins bind ubiquitinated substrates via their C-terminal UBA domain104,105, and associate with the proteasome via their N-terminal UBL domain95,106,107. This UBL-mediated interaction is carried out through binding to Rpn195, Rpn1314 or Rpn10108,109.
Although Dsk2 serves as a shuttle of ubiquitinated substrates to the proteasome, it has been surprisingly shown that its overexpression impairs proteolysis and exerts a cytotoxic effect110,111,112. This effect was shown to be attenuated by the binding of Rpn10 UIM (in its proteasome-unbound form) to Dsk2 UBL112. This association is believed to be regulated through Rpn10 monoubiquitination113, resembling the mechanism that regulates UIM binding with ubiquitinated substrates82,102,103.
Ubiquilins, the mammalian orthologs of the yeast Dsk2, are a family of four ubiquitin-like proteins that function as shuttle proteins targeting ubiquitinated proteins to the proteasome. This family is well conserved in frog, rat and human. Structurally, they harbor an N-terminal UBL domain by which they bind proteasomal Rpn10, and a C-terminal UBA domain which binds polyubiquitinated proteins114,115. Ubiquilins have been shown to mediate the removal of damaged proteins following oxidative stress116. Abnormalities in ubiquilins, such as a compromised ability to bind Rpn10, were linked to elevated cellular levels of ubiquitinated proteins, leading to aggregate formation, which in turn was implicated in the pathogenesis of several neurodegenerative disorders (e.g., amyotrophic lateral sclerosis (ALS), Huntington's and Alzheimer's diseases117,118,119).
Rad23 contains two UBA domains: a centrally located UBA1 and a C-terminal UBA2 that bind mono- and polyubiquitinated substrates with different affinities. UBA1 binds K63-linked polyubiquitin chains with a slightly higher affinity than K48-linked chains, whereas the UBA2 domain preferentially binds K48-polyubiquitin chains120. Rad23 UBL domain binds Rpn115,95, positioning it at the center of the 19S base, close to the CP's entrance121. It has been suggested that the human orthologs of Rad23 (hHR23A and hHR23B) interact with Rpn10122. As for the two yeast proteins, while it appears that they also interact with Rpn1015, a previous report suggests otherwise122. Rad23 interaction with the proteasome is inhibited by phosphorylation of its UBL domain, thereby affecting its activity123. Rad23 UBL domain was also found to bind other proteins. For example, its binding to the ubiquitin chain elongation factor Ufd2 facilitates proteasomal degradation of ubiquitin-fusion degradation (UFD) substrates124, whereas its binding to the peptidyl tRNA hydrolase Pth2 antagonizes ubiquitin-dependent proteolysis, possibly by preventing association of Rad23 with the proteasome122. Rad23's role in degradation is controversial, with a number of studies suggesting that it acts as an inhibitor125,126, while others suggesting it promotes degradation by acting as a shuttle factor targeting proteins to the proteasome15,101,106. However, these studies clearly demonstrate that ubiquitinated proteins bound to Rad23 are protected from the modification of their ubiquitin chains, i.e., elongation and deubiquitination. This stabilization effect is hypothesized to mediate efficient substrate targeting to the proteasome by inhibiting unnecessary processing of the ubiquitin chain127. Rad23 also participates in ER-associated degradation (ERAD) by binding of its Rad4-binding domain to the deglycosylase Png1, forming a complex which mediates proteasomal degradation of a specific set of ER proteins128.
Even though UBL-UBA proteins interact with the proteasome, they are able to escape degradation. Several studies in yeast showed that the C-terminal UBA domain of both Dsk2 and Rad23 is responsible for their stability129,130, presumably by preventing initiation of their proteasomal degradation130. Introduction of long unstructured stretch (which serve as initiation sites for degradation) to Rad23 C-terminus, abolishes the protective effect of its UBA domain. However, when the C-terminal UBA domain of Rad23 (UBA2) was inserted downstream of the unstructured stretch, the protective effect was re-established. This effect was specific to UBA2, as insertion of the internal UBA domain (UBA1) downstream to the unstructured sequence retained no protective effect. Furthermore, introduction of UBA2 to the C-terminus of other substrates containing an unstructured region, diminished their degradation. Taken together, these findings demonstrate that UBA-mediated protection is dependent on its localization relative to the C-terminus of the harboring proteins130.
In addition to the proteasomal intrinsic ubiquitin receptors and the non-proteasomal UBL-UBA shuttle proteins, there are also other proteins that have been implicated in shuttling of ubiquitinated substrates to the 26S proteasome. p97/VCP/Cdc48 is a highly conserved hexameric ATPase involved in numerous cellular functions, including DNA synthesis and repair, membrane fusion, disassembly of mitotic spindle, autophagic- and proteasome-mediated proteolysis, and ERAD131. Unlike other shuttle proteins which bind both to ubiquitin and to the proteasome in an ATP-independent manner, p97 hydrolyses ATP and uses the resulting energy to structurally remodel or unfold its substrates, thus separating them from complexes, extracting them from cellular structures, or generating initial unstructured segments to facilitate degradation by the proteasome. The association of p97 with ubiquitinated substrates is mediated by several ubiquitin adaptors which recognize both p97 and the substrate131,132. Several studies have demonstrated a rather more complex function of the adaptors, including binding of p97 to E3 ubiquitin ligases that ubiquitinate substrates, and an interaction with other ubiquitin-like modifiers133. One important type of p97 cofactors includes downstream processing proteins such as DUBs. They can either inhibit degradation of a given substrate by removing its ubiquitin moieties, or promote degradation by “editing” the substrates' ubiquitin chains to a length more suitable for proteasomal targeting127,132. This suggests that p97 determines the fate of extracted proteins by playing a pivotal role in their ubiquitin-dependent degradation134.
Sequestosome 1/p62 is a ubiquitin shuttling protein135 that binds ubiquitinated substrates via its C-terminal UBA domain, associates with the RP subunits Rpt1 and Rpn10 via its N-terminal PB1 domain136, thereby targeting proteins (e.g., tau) for proteasomal degradation136,137. p62 also acts as a ubiquitin receptor in autophagy-mediated degradation, directly binding to LC3, a known mediator of autophagosome formation138. p62's role as a ubiquitin receptor in both proteasome- and autophagy-mediated degradation of ubiquitinated proteins is also supported by the finding that decreasing endogenous p62 levels results in the accumulation of ubiquitinated proteins136.
Substrate deubiquitinaitng enzymes
During degradation, at least part of the ubiquitin chain moieties are rescued from degradation in a process mediated mostly by the deubiquitinating proteasome subunit, Rpn1117,18. Before deubiquitination of the substrate by Rpn11, two other DUBs can trim its ubiquitin chains: Uch37 and Ubp6/Usp14. Early removal of ubiquitin chains by these ATP-independent enzymes can antagonize substrate degradation, that in contrast to the activity of Rpn11139. Uch37 is linked to Rpn2 via Rpn1393 and removes ubiquitin moieties from the distal end of the chain, releasing only monoubiquitin19. Shortening of ubiquitin chains affects the substrate's affinity for the proteasome, which may rescue poorly ubiquitinated proteins from degradation140. Ubp6 binds the proteasome via Rpn120,141 and was shown to cleave single ubiquitin moieties142 as well as di- and tri-ubiquitins (and even longer oligomers)139 form substrate-anchored chains. A recent study showed that Ubp6 can also remove ubiquitin chains en bloc, and that in both yeast and human cells it prefers substrates that are ubiquitinated at multiple sites143. Ubp6 was also shown to inhibit the degradation of 26S proteasome substrates in a non-catalytic manner139. One model, trying to explain Ubp6's non-catalytic effect, suggests that it is mediated via stabilization of the substrate-bound conformation of the proteasome and allosteric interference with the binding of the incoming substrate139,144.
After a polyubiquitin chain has been removed en bloc from the substrate by Rpn1118,145, it must be further broken down to single recycling moieties. This function is mediated by IsoT/Ubp14/Usp5, which is a unique DUB that disassembles free polyubiquitin chains by hydrolyzing one ubiquitin at a time from the proximal end of the chain146,147,148. Optimal catalytic activity of this DUB was shown to require its zinc-finger ubiquitin binding domain (ZnF UBP), which recognizes the C-terminal Gly-Gly residues of an unanchored ubiquitin149. IsoT suppression was found to result in accumulation of free ubiquitin chains and stabilization of proteins, such as p53, a bona fide 26S proteasomal substrate148,150.
Different regulators of the proteasome
PI31, a proline-rich protein, was first described as an inhibitor of proteasomal activity, partially through inhibition of the binding between 19S (or PA28) regulatory particle to the 20S CP151,152. It was later suggested as a modulator of the immunoproteasome with no effect on the constitutive proteasome153. A study in D. melanogaster has shown that PI31, in complex with the SCF E3 ubiquitin ligase Nutcracker, regulates proteasome function by exerting a positive effect on the 26S activity, and a negative effect on the activity of the free 20S CP154. While this may resolve the apparent contradicting findings regarding PI31 effect on proteasome function, another study found that regardless of its level, PI31 has no effect on either proteasome content or function, and that such an effect may be specific to certain physiologic conditions or proteasome pools155.
Ecm29 is a large, 205 kDa, protein associated with the proteasome20 and regulating its function via several mechanisms. It was shown to directly inhibit proteasome activity in yeast, partially via inhibition of the 19S ATPase activity156,157. On the other hand, a positive effect on proteasome function was also described in yeast. It was found that Ecm29 supports proteasome assembly, as it stabilizes a 20S-19S intermediate in which 20S maturation is delayed due to temporary shortage of specific β subunits158. Other studies showed that Ecm29 is recruited to the 19S RP in response to oxidative stress, and induces disassembly of the 26S proteasome159,160. It was suggested that degradation of oxidized proteins is mediated mainly by the 20S rather than the 26S proteasome161,162. According to this model, Ecm29-dependent disassembly of the 26S holoenzyme serves to increase the abundance of 20S, allowing cells to cope with the stress. In mammals, Ecm29 (encoded by the KIAA0368 gene) promotes proteasome dissociation under oxidative stress163, and associates with various molecular motors and endosomal components. This association may be involved in its ability to recruit 26S proteasomes to distinct cellular locations, such as the ER and the centrosome164,165.
Besides the suggested role for free 20S CP, it is also active as part of other complexes different from the 'canonical' 26S proteasome. Proteasome regulatory particles other than the 19S, such as PA28αβ, PA28γ and PA200, bind the 20S to form different proteasomal complexes, and may thereby facilitate the degradation of certain substrates under different physiological conditions, and/or of those that are degraded less efficiently by the “canonical” proteasome and/or of those that may not need prior ubiquitination for their degradation166. For example, PA28γ mediates the degradation of the steroid receptor coactivator-3 (SRC3) and the cell-cycle regulator p21167,168,169. It was recently suggested that in several mammalian cell types, a considerable fraction of the 20S may reside in non-26S forms: either in its free form, or in complexes where it is capped by activators other than the 19S170,171.
Structural changes induced by substrate binding
In recent years, cryo-EM-based studies established the molecular architecture of the 26S proteasome172,173,174. Though these studies generated a near-atomic resolution structural models, they also implied dynamic heterogeneity rather than a single static proteasomal structure. Recent classification of a large data set provided researchers with the ability to dissect this structure, and to discern between three coexisting proteasomal conformational states, S1, S2 and S3175. Prior to this advancement, it has been suggested that conformational changes are part of the mechanism by which the proteasome deubiquitinates, unfolds, translocates, and degrades substrates176,177. The conformational state S1 is believed to represent the substrate-unbound proteasome and is strikingly abundant in some tissues178. Ubiquitinated substrates binding to S1, and the subsequent engagement of their initiation site within the ATPase pore, seems to induce conformational changes, resulting in S3 proteasomal conformation, corresponding with the substrate-bound form of the proteasome175. The S2 form probably corresponds to an intermediate/hybrid state between S1 and S3175, and in fact was not defined in a later structural model of endogenous proteasome in yeast, which describes only substrate-bound (M1) or -unbound (M2) states179.
Importantly, following substrate binding, the 19S RP translocates the substrate into the catalytic chamber of the 20S CP, while unfolding it and removing its conjugated ubiquitin. This requires opening of the α-ring gate, which is dependent on binding of C-terminal peptides of Rpt 19S subunits to the 20S surface3,4,180,181.
Proteasomal degradation foci
Besides direct regulation by various mechanisms, proteasome function is also dependent on its recruitment to specific cellular sites, where selective proteolytic activity is required.
Many neurodegenerative diseases are characterized by inclusion bodies enriched with ubiquitinated proteins and proteasomal subunits182,183,184,185,186,187,188,189,190, which raised the hypothesis that protein degradation is impaired in these disorders191. It was suggested that proteasomes are recruited to ubiquitinated aggregated proteins in order to degrade them, but for whatever reason are stalled; yet, this point is still debated192,193,194,195.
In addition to its possible involvement in the degradation of aggregated proteins, the proteasome has additional roles in neuronal function. It has been shown that proteasome recruitment to synapses supports neuroplasticity, as it regulates the local turnover of both pre- and post-synaptic proteins196,197,198. Researchers showed that the synaptic protein GluN2B, an NMDA receptor subunit, is critical for NMDA receptor function in synaptic stress and plasticity, which is important for learning and memory formation. It has been shown that GluN2B mediates proteasome synaptic anchoring, thus enhancing its local activity199.
Promyelocytic leukemia-nuclear bodies (PML-NBs) are spheres located in nuclei of many cell types. They are surrounded by the PML protein, which is also an oncogene involved in a chromosomal translocation that results in its fusion with retinoic acid receptor α (RARα), which is the underlying cause of promyelocytic leukemia. Normally, retinoic acid binds to RARα and abrogates its inhibitory effect on gene expression, thus leading to expression of proteins that mediate differentiation. The PML-RARα fusion retains RARα inhibitory effect, also in the presence of retinoic acid, thus inhibiting differentiation. Notably, the PML-RARα fusion also disrupts PML-NBs, as it dimerizes with native PML, probably adding to its deleterious effect200.
PML-NBs recruit several key regulators such as p53, DNA repair factors, and the ubiquitin-like protein SUMO. Therefore, they were suggested to take part in the regulation of several processes, such as DNA damage response, cell survival and senescence201,202. They have also been shown to contain proteasome203,204, as well as its transcription regulator Nrf2. Following SUMOylation, Nrf2 is ubiquitinated by the RNF4 E3 ubiquitin ligase and degraded by the PML-NB-localized proteasome, which represents an example for mutual regulation of the proteasome and Nrf249. PML, SUMO and RNF4 were also suggested to cooperate in the proteasomal-mediated removal of misfolded proteins in the nucleus, which were also described to co-localize with PML-NBs. PML was shown to have a SUMO ligase activity, facilitating SUMO-dependent ubiquitination by RNF4 and subsequent degradation of misfolded proteins by the proteasome205. How proteasomes are recruited into PML-NBs, and whether this recruitment regulates additional cellular activities is yet to be determined.
Another suggested focus for both basal and stress-induced proteasome activity is the centrosome, a perinuclear organelle composed of a pair of centrioles that are surrounded by pericentriolar material, and serves as a microtubule organizing center. This organelle is enriched in proteasome, ubiquitin and other regulators, all of which are recruited to the centrosome from the cytosol in response to proteasome inhibition and increase in the level of misfolded proteins206. Rpn10 has been shown to regulate centrosomal proteasome activity in neurons, thereby facilitating dendrite elaboration in rodent brain207.
Proteasomes were also shown to be tethered to ER membrane as part of their role in ERAD208,209,210, as well as to the outer mitochondrial membrane. This later association enhances the degradation of mitochondrial substrates211, and is upregulated in response to mitochondrial stress212. FK506-binding protein 38 (FKBP38) was suggested as a proteasome anchor to organellar membranes213. As is discussed above, Ecm29 is also involved in proteasome recruitment to different organelles, such as the ER and centrosome164,165.
The proteasome as a substrate for degradation
The proteasome is known to be a stable complex. Whereas much is known about its biogenesis, its degradation pathway(s) is still poorly understood.
Following induction of apoptosis in human cell lines, it was shown that the 19S proteasomal subunits Rpt5, Rpn2 and Rpn10 are cleaved by caspase-3. The cleavage of Rpn10 and Rpn2, which together connect the lid and base, results in impaired proteasome activity and accumulation of ubiquitinated proteins214. In D. melanogaster cells it was shown that caspase-3 activation results in the cleavage of α2, α4, and β4 subunits of the 20S, and the Rpt1 subunit of the 19S. Also, the PA28γ complex was identified as a caspase-3 substrate215. An additional degradation pathway of non-functional proteasomal subunits was recently identified in yeast. It was shown that free, unassembled subunits, are degraded by the UPS itself, and that ubiquitination of the subunits is essential for their degradation. The heat-shock protein Hsp42 was shown to mediate the degradation of the unassembled subunits by sorting them into cytoprotective compartments, such as insoluble protein deposits (IPOD), where they are degraded by the proteasome39.
Lysosomal degradation of the entire 26S proteasome complex was also described. Using a rat model, the accumulation of the proteasome within lysosomes was identified following leupeptin treatment or nutrient starvation. Moreover, it was postulated that the delivery of the proteasome to lysosomes is mediated by autophagy216. Nevertheless, the mechanism behind this process has remained elusive. Recent study in yeast has identified the independent targeting of the 19S and 20S sub-complexes for vacuolar degradation through autophagy upon nitrogen starvation217. The vacuolar degradation of the 20S proteasome depended on the DUB Ubp3, while that of the 19S proteasome was not. Furthermore, the elimination process of the proteasome following nitrogen starvation involved dissociation of the 19S and 20S proteasomes and their nuclear export217. In addition, the vacuolar targeting of a chemically or genetically inactivated 26S proteasome by autophagy has been recently described in A. thaliana. In this plant, Rpn10 can act also as a selective autophagy adaptor that simultaneously binds both ATG8 (LC3 in mammals, an autophagosomal receptor) and the proteasome, the ubiquitination of which is stimulated following its inhibition. Unlike proteasome engulfment induced by its own inhibition, the mechanism that underlies the vacuolar targeting of the proteasome upon nitrogen starvation in A. thaliana is independent of Rpn1071. It appears therefore that the lysosomal degradation of the 26S proteasome via autophagy pathway is an evolutionarily conserved process.
Future perspectives
Although the proteasome has been studied extensively, much has still remained unknown. While proteasomal activators, inhibitors, and PTMs are discovered frequently, the mechanisms that underlie their function are still elusive. The same is true for several of the proteasomal subunits, including the long sought after ubiquitin-binding ones. Even proteasome assembly and trafficking between cellular compartments (e.g., cytosol and nucleus) are not fully understood.
One important missing piece of information is a high resolution and dynamic structure of the proteasome along with a native ubiquitinated substrate. Such structure will provide insights on the attachment of the ubiquitin chain, the position of the substrate and its initiation of unfolding, insertion and degradation.
As the UPS is involved in nearly all cellular processes, it will be interesting to identify the one(s) that regulates proteasome biosynthesis and activity, as one may expect that changing pathophysiological conditions may affect the proteasome as well, most probably via direct regulatory relationships. Importantly, even for conditions known to regulate/affect proteasome biogenesis, assembly or function, a detailed mediating mechanism is still missing.
References
Tomko RJ, Hochstrasser M . Molecular architecture and assembly of the eukaryotic proteasome. Annu Rev Biochem 2013; 82:415–445.
Groll M, Bajorek M, Köhler A, et al. A gated channel into the proteasome core particle. Nat Struct Biol 2000; 7:1062–1067.
Rabl J, Smith DM, Yu Y, Chang SC, Goldberg AL, Cheng Y . Mechanism of gate opening in the 20S proteasome by the proteasomal ATPases. Mol Cell 2008; 30:360–368.
Smith DM, Chang SC, Park S, Finley D, Cheng Y, Goldberg AL . Docking of the proteasomal ATPases' carboxyl termini in the 20S proteasome's α ring opens the gate for substrate entry. Mol Cell 2007; 27:731–744.
Pickart CM, Cohen RE . Proteasomes and their kin: proteases in the machine age. Nat Rev Mol Cell Biol 2004; 5:177–187.
Groll M, Ditzel L, Löwe J, et al. Structure of 20S proteasome from yeast at 2.4 A resolution. Nature 1997; 386:463–471.
Nederlof PM, Wang HR, Baumeister W . Nuclear localization signals of human and Thermoplasma proteasomal α subunits are functional in vitro. Proc Natl Acad Sci USA 1995; 92:12060–12064.
Tanaka K, Yoshimura T, Tamura T, Fujiwara T, Kumatori A, Ichihara A . Possible mechanism of nuclear translocation of proteasomes. FEBS Lett 1990; 271:41–46.
Ogiso Y, Tomida A, Tsuruo T . Nuclear localization of proteasomes participates in stress-inducible resistance of solid tumor cells to topoisomerase II-directed drugs. Cancer Res 2002; 62:5008–5012.
Dick TP, Nussbauma K, Deeg M, et al. Contribution of proteasomal β-subunits to the cleavage of peptide substrates analyzed with yeast mutants. J Biol Chem 1998; 273:25637–25646.
Dahlmann B . Mammalian proteasome subtypes: Their diversity in structure and function. Arch Biochem Biophys 2016; 591:132–140.
Díaz-Villanueva JF, Díaz-Molina R, García-González V . Protein folding and mechanisms of proteostasis. Int J Mol Sci 2015; 16:17193–17230.
Heinemeyer W, Ramos PC, Dohmen RJ . Ubiquitin-proteasome system. Cell Mol Life Sci 2004; 61:1562–1578.
Husnjak K, Elsasser S, Zhang N, et al. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature 2008; 453:481–488.
Shi Y, Chen X, Elsasser S, et al. Rpn1 provides adjacent receptor sites for substrate binding and deubiquitination by the proteasome. Science 2016; 351:aad9421.1–10.
Fu H, Sadis S, Rubin DM, et al. Multiubiquitin chain binding and protein degradation are mediated by distinct domains within the 26 S proteasome subunit Mcb1. J Biol Chem 1998; 273:1970–1981.
Verma R, Aravind L, Oania R, et al. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science 2002; 298:611–615.
Yao T, Cohen RE . A cryptic protease couples deubiquitination and degradation by the proteasome. Nature 2002; 419:403–407.
Lam YA, Xu W, DeMartino GN, Cohen RE . Editing of ubiquitin conjugates by an isopeptidase in the 26S proteasome. Nature 1997; 385:737–740.
Leggett DS, Hanna J, Borodovsky A, et al. Multiple associated proteins regulate proteasome structure and function. Mol Cell 2002; 10:495–507.
Maytal-Kivity V, Reis N, Hofmann K, Glickman MH . MPN+, a putative catalytic motif found in a subset of MPN domain proteins from eukaryotes and prokaryotes, is critical for Rpn11 function. BMC Biochem 2002; 3:28.
Kleijnen MF, Roelofs J, Park S, et al. Stability of the proteasome can be regulated allosterically through engagement of its proteolytic active sites. Nat Struct Mol Biol 2007; 14:1180–1188.
Li X, Demartino GN, Craiu A, et al. Variably modulated gating of the 26S proteasome by ATP and polyubiquitin. Biochem J 2009; 421:397–404.
Gallastegui N, Groll M . The 26S proteasome: assembly and function of a destructive machine. Trends Biochem Sci 2010; 35:634–642.
Finley D, Chen X, Walters KJ . Gates, channels, and switches: elements of the proteasome machine. Trends Biochem Sci 2015; 41:77–93.
Hirano Y, Hendil KB, Yashiroda H, et al. A heterodimeric complex that promotes the assembly of mammalian 20S proteasomes. Nature 2005; 437:1381–1385.
Stadtmueller BM, Kish-Trier E, Ferrell K, et al. Structure of a proteasome Pba1-Pba2 complex implications for proteasome assembly, activation, and biological function. J Biol Chem 2012; 287:37371–37382.
Hirano Y, Hayashi H, Iemura S, et al. Cooperation of multiple chaperones required for the assembly of mammalian 20S proteasomes. Mol Cell 2006; 24:977–984.
Murata S, Yashiroda H, Tanaka K . Molecular mechanisms of proteasome assembly. Nat Rev Mol Cell Biol 2009; 10:104–115.
Fricke B, Heink S, Steffen J, Kloetzel P-M, Krüger E . The proteasome maturation protein POMP facilitates major steps of 20S proteasome formation at the endoplasmic reticulum. EMBO Rep 2007; 8:1170–1175.
Ramos PC, Höckendorff J, Johnson ES, Varshavsky A, Dohmen RJ . Ump1p is required for proper maturation of the 20S proteasome and becomes its substrate upon completion of the assembly. Cell 1998; 92:489–499.
Bar-Nun S, Glickman MH . Proteasomal AAA-ATPases: structure and function. Biochim Biophys Acta 2012; 1823:67–82.
Hanssum A, Zhong Z, Rousseau A, Krzyzosiak A, Sigurdardottir A, Bertolotti A . An inducible chaperone adapts proteasome assembly to stress. Mol Cell 2014; 55:566–577.
Saeki Y, Toh-E A, Kudo T, Kawamura H, Tanaka K . Multiple proteasome-interacting proteins assist the assembly of the yeast 19S regulatory particle. Cell 2009; 137:900–913.
Tomko RJ, Taylor DW, Chen ZA, Wang H-W, Rappsilber J, Hochstrasser M . A single α helix drives extensive remodeling of the proteasome lid and completion of regulatory particle assembly. Cell 2015; 163:432–444.
Imai J, Maruya M, Yashiroda H, Yahara I, Tanaka K . The molecular chaperone Hsp90 plays a role in the assembly and maintenance of the 26S proteasome. EMBO J 2003; 22:3557–3567.
Piterman R, Braunstein I, Isakov E, et al. Vwa Domain of S5a restricts the ability to bind ubiquitin and Ubl to the 26S proteasome. Mol Biol Cell 2014; 25:3988–3998.
Hendil KB, Hartmann-Petersen R, Tanaka K . 26 S proteasomes function as stable entities. J Mol Biol 2002; 315:627–636.
Peters LZ, Karmon O, David-Kadoch G, et al. The protein quality control machinery regulates its misassembled proteasome subunits. PLoS Genet 2015; 11:e1005178.
Meiners S, Heyken D, Weller A, et al. Inhibition of proteasome activity induces concerted expression of proteasome genes and de novo formation of mammalian proteasomes. J Biol Chem 2003; 278:21517–21525.
Zhang Y, Manning BD . mTORC1 signaling activates NRF1 to increase cellular proteasome levels. Cell Cycle 2015; 14:2011–2017.
Sha Z, Goldberg AL . Proteasome-mediated processing of Nrf1 is essential for coordinate induction of all proteasome subunits and p97. Curr Biol 2014; 24:1573–1583.
Mitsiades N, Mitsiades CS, Poulaki V, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci USA 2002; 99:14374–14379.
Lundgren J, Masson P, Mirzaei Z, Young P . Identification and characterization of a Drosophila proteasome regulatory network. Mol Cell Biol 2005; 25:4662–4675.
Li X, Matilainen O, Jin C, Glover-Cutter KM, Holmberg CI, Blackwell TK . Specific SKN-1/NrF stress responses to perturbations in translation elongation and proteasome activity. PLoS Genet 2011; 7:9–11.
London MK, Keck BI, Ramos PC, Dohmen RJ . Regulatory mechanisms controlling biogenesis of ubiquitin and the proteasome. FEBS Lett 2004; 567:259–264.
Radhakrishnan SK, Lee CS, Young P, Beskow A, Chan JY, Deshaies RJ . Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol Cell 2010; 38:17–28.
Steffen J, Seeger M, Koch A, Krüger E . Proteasomal degradation is transcriptionally controlled by TCF11 via an ERAD-dependent feedback loop. Mol Cell 2010; 40:147–158.
Malloy MT, McIntosh DJ, Walters TS, Flores A, Goodwin JS, Arinze IJ . Trafficking of the transcription factor Nrf2 to promyelocytic leukemia-nuclear bodies: Implications for degradation of nrf2 in the nucleus. J Biol Chem 2013; 288:14569–14583.
Kwak MK, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW . Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol 2003; 23:8786–8794.
Motohashi H, Yamamoto M . Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med 2004; 10:549–557.
Pickering AM, Staab TA, Tower J, Sieburth DS, Davies KJ . A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative-stress adaptation in mammals, C. elegans and D. melanogaster. J Exp Biol 2013; 216:543–553.
Choe KP, Przybysz AJ, Strange K . The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol Cell Biol 2009; 29:2704–2715.
Wang L, Mao X, Ju D, Xie Y . Rpn4 is a physiological substrate of the Ubr2 ubiquitin ligase. J Biol Chem 2004; 279:55218–55223.
Ju D, Xie Y . Proteasomal degradation of RPN4 via two distinct mechanisms, ubiquitin-dependent and -independent. J Biol Chem 2004; 279:23851–23854.
Xie Y, Varshavskya . RPN4 is a ligand, substrate, and transcriptional regulator of the 26S proteasome: a negative feedback circuit. Proc Natl Acad Sci USA 2001; 98:3056–3061.
Mannhaupt G, Schnall R, Karpov V, Vetter I, Feldmann H . Rpn4p acts as a transcription factor by binding to PACE, a nonamer box found upstream of 26S proteasomal and other genes in yeast. FEBS Lett 1999; 450:27–34.
Ma M, Liu ZL . Comparative transcriptome profiling analyses during the lag phase uncover YAP1, PDR1, PDR3, RPN4, and HSF1 as key regulatory genes in genomic adaptation to the lignocellulose derived inhibitor HMF for Saccharomyces cerevisiae. BMC Genomics 2010; 11:660.
Chondrogianni N, Tzavelas C, Pemberton AJ, Nezis IP, Rivett AJ, Gonos ES . Overexpression of proteasome β5 subunit increases the amount of assembled proteasome and confers ameliorated response to oxidative stress and higher survival rates. J Biol Chem 2005; 280:11840–11850.
Vilchez D, Morantte I, Liu Z, et al. RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature 2012; 489:263–268.
Vilchez D, Boyer L, Morantte I, et al. Increased proteasome activity in human embryonic stem cells is regulated by PSMD11. Nature 2012; 489:304–308.
Laplante M, Sabatini DM . mTOR signaling in growth control and disease. Cell 2013; 149:274–293.
Zhang Y, Nicholatos J, Dreier JR, et al. Coordinated regulation of protein synthesis and degradation by mTORC1. Nature 2014; 513:440–443.
Zhao J, Zhai B, Gygi SP, Goldberg AL . mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proc Natl Acad Sci USA 2015; 112:15790–15797.
Zhao J, Garcia GA, Goldberg AL . Control of proteasomal proteolysis by mTOR. Nature 2016; 529:E1–E2.
Zhang Y, Manning BD . Zhang & Manning reply. Nature 2016; 529:E2–E3.
Hirano H, Kimura Y, Kimura A . Biological significance of co- and post-translational modifications of the yeast 26S proteasome. J Proteomics 2015; 134:37–46.
Zong N, Ping P, Lau E, et al. Lysine ubiquitination and acetylation of human cardiac 20S proteasomes. Proteomics Clin Appl 2014; 8:590–594.
Guo X, Engel JL, Xiao J, et al. UBLCP1 is a 26S proteasome phosphatase that regulates nuclear proteasome activity. Proc Natl Acad Sci USA 2011; 108:18649–18654.
van Deventer S, Menendez-Benito V, van Leeuwen F, Neefjes J . N-terminal acetylation and replicative age affect proteasome localization and cell fitness during aging. J Cell Sci 2015; 128:109–117.
Marshall RS, Li F, Gemperline DC, Book AJ, Vierstra RD . Autophagic degradation of the 26S proteasome is mediated by the dual ATG8/ubiquitin receptor RPN10 in Arabidopsis. Mol Cell 2015; 58:1053–1066.
Iwafune Y, Kawasaki H, Hirano H . Electrophoretic analysis of phosphorylation of the yeast 20S proteasome. Electrophoresis 2002; 23:329–338.
Bose S, Stratford FLL, Broadfoot KI, Mason GGF, Rivett AJ . Phosphorylation of 20S proteasome α subunit C8 (α7) stabilizes the 26S proteasome and plays a role in the regulation of proteasome complexes by γ-interferon. Biochem J 2004; 378:177–184.
Kikuchi J, Iwafune Y, Akiyama T, et al. Co- and post-translational modifications of the 26S proteasome in yeast. Proteomics 2010; 10:2769–2779.
Zhang F, Hu Y, Huang P, Toleman CA, Paterson AJ, Kudlow JE . Proteasome function is regulated by cyclic AMP-dependent protein kinase through phosphorylation of Rpt6. J Biol Chem 2007; 282:22460–22471.
Satoh K, Sasajima H, Nyoumura K, Yokosawa H, Sawada H . Assembly of the 26S proteasome is regulated by phosphorylation of the p45/Rpt6 ATPase subunit. Biochemistry 2001; 40:314–319.
Lu H, Zong C, Wang Y, et al. Revealing the dynamics of the 20S proteasome phosphoproteome: a combined CID and electron transfer dissociation approach. Mol Cell Proteomics 2008; 7:2073–2089.
Ramnanan CJ, Allan ME, Groom AG, Storey KB . Regulation of global protein translation and protein degradation in aerobic dormancy. Mol Cell Biochem 2009; 323:9–20.
Lee S-H, Park Y, Yoon SK, Yoon J-B . Osmotic stress inhibits proteasome by p38 MAPK-dependent phosphorylation. J Biol Chem 2010; 285:41280–41289.
Liu X, Huang W, Li C, et al. Interaction between c-Abl and Arg tyrosine kinases and proteasome subunit PSMA7 regulates proteasome degradation. Mol Cell 2006; 22:317–327.
Lokireddy S, Kukushkin NV, Goldberg AL . cAMP-induced phosphorylation of 26S proteasomes on Rpn6/PSMD11 enhances their activity and the degradation of misfolded proteins. Proc Natl Acad Sci USA 2015; 112:E7176–7185.
Isasa M, Katz EJ, Kim W, et al. Monoubiquitination of RPN10 regulates substrate recruitment to the proteasome. Mol Cell 2010; 38:733–745.
Besche HC, Sha Z, Kukushkin N V, et al. Autoubiquitination of the 26S proteasome on Rpn13 regulates breakdown of ubiquitin conjugates. EMBO J 2014; 33:1159–1176.
Wang D, Fang C, Zong NC, et al. Regulation of acetylation restores proteolytic function of diseased myocardium in mouse and human. Mol Cell Proteomics 2013; 12:3793–3802.
Silva GM, Netto LES, Simões V, et al. Redox control of 20S proteasome gating. Antioxid Redox Signal 2012; 16:1183–1194.
Kimura A, Kurata Y, Nakabayashi J, Kagawa H, Hirano H . N-myristoylation of the Rpt2 subunit of the yeast 26S proteasome is implicated in the subcellular compartment-specific protein quality control system. J Proteomics 2016; 130:33–41.
Zhang F, Su K, Yang X, Bowe DB, Paterson AJ, Kudlow JE . O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell 2003; 115:715–725.
Ullrich O, Reinheckel T, Sitte N, Hass R, Grune T, Davies KJ . Poly-ADP ribose polymerase activates nuclear proteasome to degrade oxidatively damaged histones. Proc Natl Acad Sci USA 1999; 96:6223–6228.
Lam YA, Lawson TG, Velayutham M, Zweier JL, Pickart CM . A proteasomal ATPase subunit recognizes the polyubiquitin degradation signal. Nature 2002; 416:763–767.
Paraskevopoulos K, Kriegenburg F, Tatham MH, et al. Dss1 is a 26S proteasome ubiquitin receptor. Mol Cell 2014; 56:453–461.
Schreiner P, Chen X, Husnjak K, et al. Ubiquitin docking at the proteasome through a novel pleckstrin-homology domain interaction. Nature 2008; 453:548–552.
Yao T, Song L, Xu W, et al. Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nat Cell Biol 2006; 8:994–1002.
Hamazaki J, Iemura S-I, Natsume T, Yashiroda H, Tanaka K, Murata S . A novel proteasome interacting protein recruits the deubiquitinating enzyme UCH37 to 26S proteasomes. EMBO J 2006; 25:4524–4536.
Rosenzweig R, Bronner V, Zhang D, Fushman D, Glickman MH . Rpn1 and Rpn2 coordinate ubiquitin processing factors at proteasome. J Biol Chem 2012; 287:14659–14671.
Elsasser S, Gali RR, Schwickart M, et al. Proteasome subunit Rpn1 binds ubiquitin-like protein domains. Nat Cell Biol 2002; 4:725–730.
Gomez TA, Kolawa N, Gee M, Sweredoski MJ, Deshaies RJ . Identification of a functional docking site in the Rpn1 LRR domain for the UBA-UBL domain protein Ddi1. BMC Biol 2011; 9:33.
Haracska L, Udvardy A . Mapping the ubiquitin-binding domains in the p54 regulatory complex subunit of the Drosophila 26S protease. FEBS Lett 1997; 412:331–336.
van Nocker S, Sadis S, Rubin DM, et al. The multiubiquitin-chain-binding protein Mcb1 is a component of the 26S proteasome in Saccharomyces cerevisiae and plays a nonessential, substrate-specific role in protein turnover. Mol Cell Biol 1996; 16:6020–6028.
van Nocker S, Deveraux Q, Rechsteiner M, Vierstra RD . Arabidopsis MBP1 gene encodes a conserved ubiquitin recognition component of the 26S proteasome. Proc Natl Acad Sci USA 1996; 93:856–860.
Lipinszki Z, Kiss P, Pál M, et al. Developmental-stage-specific regulation of the polyubiquitin receptors in Drosophila melanogaster. J Cell Sci 2009; 122:3083–3092.
Verma R, Oania R, Graumann J, Deshaies RJ . Multiubiquitin chain receptors define a layer of substrate selectivity in the ubiquitin-proteasome system. Cell 2004; 118:99–110.
Woelk T, Oldrini B, Maspero E, et al. Molecular mechanisms of coupled monoubiquitination. Nat Cell Biol 2006; 8:1246–1254.
Di Fiore PP, Polo S, Hofmann K . When ubiquitin meets ubiquitin receptors: a signaling connection. Nat Rev Mol Cell Biol 2003; 4:491–497.
Hofmann K, Bucher P . The UBA domain: A sequence motif present in multiple enzyme classes of the ubiquitination pathway. Trends Biochem Sci 1996; 21:172–173.
Wilkinson CR, Seeger M, Hartmann-Petersen R, et al. Proteins containing the UBA domain are able to bind to multi-ubiquitin chains. Nat Cell Biol 2001; 3:939–943.
Elsasser S, Chandler-Mitilello D, Müller B, Hanna J, Finley D . Rad23 and Rpn10 serve as alternate ubiquitin receptors for the proteasome. J Biol Chem 2004; 279:26817–26822.
Schauber C, Chen L, Tongaonkar P, et al. Rad23 links DNA repair to the ubiquitin/proteasome pathway. Nature 1998; 391:715–718.
Hiyama H, Yokoi M, Masutani C, et al. Interaction of hHR23 with S5a. The ubiquitin-like domain of hHR23 mediates interaction with S5a subunit of 26S proteasome. J Biol Chem 1999; 274:28019–28025.
Walters KJ, Kleijnen MF, Goh AM, Wagner G, Howley PM . Structural studies of the interaction between ubiquitin family proteins and proteasome subunit S5a. Biochemistry 2002; 41:1767–1777.
Funakoshi M, Sasaki T, Nishimoto T, Kobayashi H . Budding yeast Dsk2p is a polyubiquitin-binding protein that can interact with the proteasome. Proc Natl Acad Sci USA 2002; 99:745–750.
Biggins S, Ivanovska I, Rose MD . Yeast ubiquitin-like genes are involved in duplication of the microtubule organizing center. J Cell Biol 1996; 133:1331–1346.
Matiuhin Y, Kirkpatrick DS, Ziv I, et al. Extraproteasomal Rpn10 restricts access of the polyubiquitin-binding protein Dsk2 to proteasome. Mol Cell 2008; 32:415–425.
Zuin A, Bichmann A, Isasa M, Puig-Sàrries P, Díaz LM, Crosas B . Rpn10 monoubiquitination orchestrates the association of the ubiquilin-type DSK2 receptor with the proteasome. Biochem J 2015; 472:353–365.
Ko HS, Uehara T, Tsuruma K, Nomura Y . Ubiquilin interacts with ubiquitylated proteins and proteasome through its ubiquitin-associated and ubiquitin-like domains. FEBS Lett 2004; 566:110–114.
Hamazaki J, Hirayama S, Murata S . Redundant roles of Rpn10 and Rpn13 in recognition of ubiquitinated proteins and cellular homeostasis. PLoS Genet 2015; 11:1–20.
Liu Y, Lü L, Hettinger CL, et al. Ubiquilin-1 protects cells from oxidative stress and ischemic stroke caused tissue injury in mice. J Neurosci 2014; 34:2813–2821.
Wang H, Monteiro MJ . Ubiquilin interacts and enhances the degradation of expanded-polyglutamine proteins. Biochem Biophys Res Commun 2007; 360:423–427.
Wang H, Lim PJ, Yin C, Rieckher M, Vogel BE, Monteiro MJ . Suppression of polyglutamine-induced toxicity in cell and animal models of Huntington's disease by ubiquilin. Hum Mol Genet 2006; 15:1025–1041.
Haapasalo A, Viswanathan J, Kurkinen KM, et al. Involvement of ubiquilin-1 transcript variants in protein degradation and accumulation. Commun Integr Biol 2011; 4:428–432.
Raasi S, Varadan R, Fushman D, Pickart CM . Diverse polyubiquitin interaction properties of ubiquitin-associated domains. Nat Struct Mol Biol 2005; 12:708–714.
Rosenzweig R, Osmulski PA, Gaczynska M, Glickman MH . The central unit within the 19S regulatory particle of the proteasome. Nat Struct Mol Biol 2008; 15:573–580.
Ishii T, Funakoshi M, Kobayashi H . Yeast Pth2 is a UBL domain-binding protein that participates in the ubiquitin-proteasome pathway. EMBO J 2006; 25:5492–5503.
Liang RY, Chen L, Ko BT, et al. Rad23 interaction with the proteasome is regulated by phosphorylation of its ubiquitin-like (UbL) domain. J Mol Biol 2014; 426:4049–4060.
Kim I, Mi K, Rao H . Multiple interactions of rad23 suggest a mechanism for ubiquitylated substrate delivery important in proteolysis. Mol Biol Cell 2004; 15:3357–3365.
Ortolan TG, Tongaonkar P, Lambertson D, Chen L, Schauber C, Madura K . The DNA repair protein rad23 is a negative regulator of multi-ubiquitin chain assembly. Nat Cell Biol 2000; 2:601–608.
Raasi S, Pickart CM . Rad23 ubiquitin-associated domains (UBA) inhibit 26S proteasome-catalyzed proteolysis by sequestering lysine 48-linked polyubiquitin chains. J Biol Chem 2003; 278:8951–8959.
Richly H, Rape M, Braun S, Rumpf S, Hoege C, Jentsch S . A series of ubiquitin binding factors connects CDC48/p97 to substrate multiubiquitylation and proteasomal targeting. Cell 2005; 120:73–84.
Kim I, Ahn J, Liu C, et al. The Png1-Rad23 complex regulates glycoprotein turnover. J Cell Biol 2006; 172:211–219.
Heessen S, Masucci MG, Dantuma NP . The UBA2 domain functions as an intrinsic stabilization signal that protects rad23 from proteasomal degradation. Mol Cell 2005; 18:225–235
Heinen C, Acs K, Hoogstraten D, Dantuma NP . C-terminal UBA domains protect ubiquitin receptors by preventing initiation of protein degradation. Nat Commun 2011; 2:191.
Meyer H, Bug M, Bremer S . Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol 2012; 14:117–123.
Stolz A, Hilt W, Buchberger A, Wolf DH . Cdc48: a power machine in protein degradation. Trends Biochem Sci 2011; 36:515–523.
Alexandru G, Graumann J, Smith GT, Kolawa NJ, Fang R, Deshaies RJ . UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1α turnover. Cell 2008; 134:804–816.
Jentsch S, Rumpf S . Cdc48 (p97): a “molecular gearbox” in the ubiquitin pathway? Trends Biochem Sci 2007; 32:6–11.
Vadlamudi RK, Joung I, Strominger JL, Shin J . p62, a phosphotyrosine-independent ligand of the SH2 domain of p56 lck, belongs to a new class of ubiquitin-binding proteins. J Biol Chem 1996; 271:20235–20237.
Seibenhener M, Babu J . Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol 2004; 24:8055–8068.
Babu JR, Geetha T, Wooten MW . Sequestosome 1/p62 shuttles polyubiquitinated tau for proteasomal degradation. J Neurochem 2005; 94:192–203.
Pankiv S, Clausen TH, Lamark T, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 2007; 282:24131–24145.
Hanna J, Hathaway NA, Tone Y, et al. Deubiquitinating enzyme Ubp6 functions noncatalytically to delay proteasomal degradation. Cell 2006; 127:99–111.
Lam YA, Demartino GN, Pickart CM, Cohen RE, Chem JB . Specificity of the ubiquitin isopeptidase in the PA700 regulatory complex of 26S proteasomes. J Biol Chem 1997; 272:28438–28446.
Aufderheide A, Beck F, Stengel F, et al. Structural characterization of the interaction of Ubp6 with the 26S proteasome. Proc Natl Acad Sci USA 2015; 112:8626–8631.
Hu M, Li P, Song L, et al. Structure and mechanisms of the proteasome-associated deubiquitinating enzyme USP14. EMBO J 2005; 24:3747–3756.
Lee BH, Lu Y, Prado MA, et al. USP14 deubiquitinates proteasome-bound substrates that are ubiquitinated at multiple sites. Nature 2016; 532:398–401.
Bashore C, Dambacher CM, Goodall EA, Matyskiela ME, Lander GC, Martin A . Ubp6 deubiquitinase controls conformational dynamics and substrate degradation of the 26S proteasome. Nat Struct Mol Biol 2015; 22:712–719.
Verma R, Aravind L, Oania R, et al. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science 2002; 298:611–615.
Wilkinson KD, Tashayev VL, O'Connor LB, Larsen CN, Kasperek E, Pickart CM . Metabolism of the polyubiquitin degradation signal: structure, mechanism, and role of isopeptidase T. Biochemistry 1995; 34:14535–14546.
Hadari T, Warms JVB, Rose IA, Hershko A . A ubiquitin C-terminal isopeptidase that acts on polyubiquitin chains: Role in protein degradation. J Biol Chem 1992; 267:719–727.
Amerik Ay, Swaminathan S, Krantz BA, Wilkinson KD, Hochstrasser M . In vivo disassembly of free polyubiquitin chains by yeast Ubp14 modulates rates of protein degradation by the proteasome. EMBO J 1997; 16:4826–4838.
Reyes-Turcu FE, Horton JR, Mullally JE, Heroux A, Cheng X, Wilkinson KD . The ubiquitin binding domain ZnF UBP recognizes the C-terminal diglycine motif of unanchored ubiquitin. Cell 2006; 124:1197–1208.
Dayal S, Sparks A, Jacob J, Allende-Vega N, Lane DP, Saville MK . Suppression of the deubiquitinating enzyme USP5 causes the accumulation of unanchored polyubiquitin and the activation of p53. J Biol Chem 2009; 284:5030–5041.
Zaiss DMW, Standera S, Holzhütter H, Kloetzel PM, Sijts AJAM . The proteasome inhibitor PI31 competes with PA28 for binding to 20S proteasomes. FEBS Lett 1999; 457:333–338.
McCutchen-Maloney SL, Matsuda K, Shimbara N, et al. cDNA cloning, expression, and functional characterization of PI31, a proline-rich inhibitor of the proteasome. J Biol Chem 2000; 275:18557–18565.
Zaiss DMW, Standera S, Kloetzel PM, Sijts AJAM . PI31 is a modulator of proteasome formation and antigen processing. Proc Natl Acad Sci USA 2002; 99:14344–14349.
Bader M, Benjamin S, Wapinski OL, Smith DM, Goldberg AL, Steller H . A conserved F box regulatory complex controls proteasome activity in Drosophila. Cell 2011; 145:371–382.
Li X, Thompson D, Kumar B, DeMartino GN . Molecular and cellular roles of PI31 (PSMF1) protein in regulation of proteasome function. J Biol Chem 2014; 289:17392–17405.
Lee SYC, De La Mota-Peynado A, Roelofs J . Loss of Rpt5 protein interactions with the core particle and Nas2 protein causes the formation of faulty proteasomes that are inhibited by Ecm29 protein. J Biol Chem 2011; 286:36641–36651.
De La Mota-Peynado A, Lee SYC, Pierce BM, Wani P, Singh CR, Roelofs J . The proteasome-associated protein Ecm29 inhibits proteasomal ATPase activity and in vivo protein degradation by the proteasome. J Biol Chem 2013; 288:29467–29481.
Lehmann A, Niewienda A, Jechow K, Janek K, Enenkel C . Ecm29 fulfils quality control functions in proteasome assembly. Mol Cell 2010; 38:879–888.
Park S, Kim W, Tian G, Gygi SP, Finley D . Structural defects in the regulatory particle-core particle interface of the proteasome induce a novel proteasome stress response. J Biol Chem 2011; 286:36652–36666.
Wang X, Yen J, Kaiser P, Huang L . Regulation of the 26S proteasome complex during oxidative stress. Sci Signal 2010; 3:ra88.
Davies KJ a . Degradation of oxidized proteins by the 20S proteasome. Biochimie 2001; 83:301–310.
Breusing N, Grune T . Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol Chem 2008; 389:203–209.
Haratake K, Sato A, Tsuruta F, Chiba T . KIAA0368-deficiency affects disassembly of 26S proteasome under oxidative stress condition. J Biochem 2016; 159:609–618.
Gorbea C, Goellner GM, Teter K, Holmes RK, Rechsteiner M . Characterization of mammalian Ecm29, a 26S proteasome-associated protein that localizes to the nucleus and membrane vesicles. J Biol Chem 2004; 279:54849–54861.
Gorbea C, Pratt G, Ustrell V, et al. A protein interaction network for Ecm29 links the 26S proteasome to molecular motors and endosomal components. J Biol Chem 2010; 285:31616–31633.
Kish-Trier E, Hill CP . Structural biology of the proteasome. Annu Rev Biophys 2013; 42:29–49.
Li X, Lonard DM, Jung SY, et al. The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGγ proteasome. Cell 2006; 124:381–392.
Li X, Amazit L, Long W, Lonard DM, Monaco JJ, O'Malley BW . Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGγ-proteasome pathway. Mol Cell 2007; 26:831–842.
Chen X, Barton LF, Chi Y, Clurman BE, Roberts JM . Ubiquitin-independent degradation of cell-cycle inhibitors by the REGγ proteasome. Mol Cell 2007; 26:843–852.
Fabre B, Lambour T, Garrigues L, et al. Deciphering preferential interactions within supramolecular protein complexes: the proteasome case. Mol Syst Biol 2015; 11:771.
Fabre B, Lambour T, Garrigues L, et al. Label-free quantitative proteomics reveals the dynamics of proteasome complexes composition and stoichiometry in a wide range of human cell lines. J Proteome Res 2014; 13:3027–3037.
Lander GC, Estrin E, Matyskiela ME, Bashore C, Nogales E, Martin A . Complete subunit architecture of the proteasome regulatory particle. Nature 2012; 482:186–191.
Beck F, Unverdorben P, Bohn S, et al. Near-atomic resolution structural model of the yeast 26S proteasome. Proc Natl Acad Sci USA 2012; 109:14870–14875.
Lasker K, Förster F, Bohn S, et al. Molecular architecture of the 26S proteasome holocomplex determined by an integrative approach. Proc Natl Acad Sci USA 2012; 109:1380–1387.
Unverdorben P, Beck F, Sledz P, et al. Deep classification of a large cryo-EM dataset defines the conformational landscape of the 26S proteasome. Proc Natl Acad Sci USA 2014; 111:5544–5549.
Matyskiela ME, Lander GC, Martin A . Conformational switching of the 26S proteasome enables substrate degradation. Nat Struct Mol Biol 2013; 20:781–788.
Śledź P, Unverdorben P, Beck F, et al. Structure of the 26S proteasome with ATP-γS bound provides insights into the mechanism of nucleotide-dependent substrate translocation. Proc Natl Acad Sci USA 2013; 110:7264–7269.
Asano S, Fukuda Y, Beck F, et al. Proteasomes. A molecular census of 26S proteasomes in intact neurons. Science 2015; 347:439–442.
Luan B, Huang X, Wu J, et al. Structure of an endogenous yeast 26S proteasome reveals two major conformational states. Proc Natl Acad Sci USA 2016; 113:2642–2647.
da Fonseca PCA, Morris EP . Structure of the human 26S proteasome: subunit radial displacements open the gate into the proteolytic core. J Biol Chem 2008; 283:23305–23314.
Gillette TG, Kumar B, Thompson D, Slaughter CA, DeMartino GN . Differential roles of the COOH termini of AAA subunits of PA700 (19S regulator) in asymmetric assembly and activation of the 26S proteasome. J Biol Chem 2008; 283:31813–31822.
Schmidt T, Lindenberg KS, Krebs A, et al. Protein surveillance machinery in brains with spinocerebellar ataxia type 3: redistribution and differential recruitment of 26S proteasome subunits and chaperones to neuronal intranuclear inclusions. Ann Neurol 2002; 51:302–310.
Stenoien DL, Cummings CJ, Adams HP, et al. Polyglutamine-expanded androgen receptors form aggregates that sequester heat shock proteins, proteasome components and SRC-1, and are suppressed by the HDJ-2 chaperone. Hum Mol Genet 1999; 8:731–741.
Schipper-Krom S, Juenemann K, Jansen AH, et al. Dynamic recruitment of active proteasomes into polyglutamine initiated inclusion bodies. FEBS Lett 2014; 588:151–159.
Davies SW, Turmaine M, Cozens BA, et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 1997; 90:537–548.
Rousseau E, Kojima R, Hoffner G, Djian P, Bertolotti A . Misfolding of proteins with a polyglutamine expansion is facilitated by proteasomal chaperones. J Biol Chem 2009; 284:1917–1929.
Cummings CJ, Mancini MA, Antalffy B, DeFranco DB, Orr HT, Zoghbi HY . Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nat Genet 1998; 19:148–154.
DiFiglia M . Aggregation of Huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 1997; 277:1990–1993.
Jana NR . Altered proteasomal function due to the expression of polyglutamine-expanded truncated N-terminal huntingtin induces apoptosis by caspase activation through mitochondrial cytochrome c release. Hum Mol Genet 2001; 10:1049–1059.
McCampbell A, Fischbeck KH . Polyglutamine and CBP: fatal attraction? Nat Med 2001; 7:528–530.
Dantuma NP, Bott LC . The ubiquitin-proteasome system in neurodegenerative diseases: precipitating factor, yet part of the solution. Front Mol Neurosci 2014; 7:70.
Maynard CJ, Böttcher C, Ortega Z, et al. Accumulation of ubiquitin conjugates in a polyglutamine disease model occurs without global ubiquitin/proteasome system impairment. Proc Natl Acad Sci USA 2009; 106:13986–13991.
Bence NF, Sampat RM, Kopito RR . Impairment of the ubiquitin-proteasome system by protein aggregation. Science 2001; 292:1552–1555.
Hipp MS, Patel CN, Bersuker K, et al. Indirect inhibition of 26S proteasome activity in a cellular model of Huntington's disease. J Cell Biol 2012; 196:573–587.
Myeku N, Clelland CL, Emrani S, et al. Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP-PKA signaling. Nat Med 2015; 22:46–53.
Hegde AN . Ubiquitin-proteasome-mediated local protein degradation and synaptic plasticity. Prog Neurobiol 2004; 73:311–357.
Pinto MJ, Alves PL, Martins L, et al. The proteasome controls presynaptic differentiation through modulation of an on-site pool of polyubiquitinated conjugates. J Cell Biol 2016; 212:789–801.
Tsai NP . Ubiquitin proteasome system-mediated degradation of synaptic proteins: An update from the postsynaptic side. Biochim Biophys Acta 2014; 1843:2838–2842.
Ferreira JS, Schmidt J, Rio P, et al. GluN2B-containing NMDA receptors regulate AMPA receptor traffic through anchoring of the synaptic proteasome. J Neurosci 2015; 35:8462–8479.
Lallemand-Breitenbach V, Zhu J, Chen Z, de Thé H . Curing APL through PML/RARA degradation by As2O3. Trends Mol Med 2012; 18:36–42.
Ivanschitz L, Takahashi Y, Jollivet F, Ayrault O, Le Bras M, de Thé H . PML IV/ARF interaction enhances p53 SUMO-1 conjugation, activation, and senescence. Proc Natl Acad Sci USA 2015; 112:14278–14283.
Lallemand-Breitenbach V, de Thé H . PML nuclear bodies. Cold Spring Harb Perspect Biol 2010; 2:a000661.
Fabunmi RP, Wigley WC, Thomas PJ, DeMartino GN . Interferon γ regulates accumulation of the proteasome activator PA28 and immunoproteasomes at nuclear PML bodies. J Cell Sci 2001; 114:29–36.
Lallemand-Breitenbach V, Zhu J, Puvion F, et al. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor α degradation. J Exp Med 2001; 193:1361–1371.
Guo L, Giasson BI, Glavis-Bloom A, et al. A cellular system that degrades misfolded proteins and protects against neurodegeneration. Mol Cell 2014; 55:15–30.
Wigley CW, Fabunmi RP, Lee MG, et al. Dynamic association of proteasomal machinery with the centrosome. J Cell Biol 1999; 145:481–490.
Puram S V, Kim AH, Park HY, Anckar J, Bonni A . The ubiquitin receptor S5a/Rpn10 links centrosomal proteasomes with dendrite development in the mammalian brain. Cell Rep 2013; 4:19–30.
Ng W, Sergeyenko T, Zeng N, Brown JD, Römisch K . Characterization of the proteasome interaction with the Sec61 channel in the endoplasmic reticulum. J Cell Sci 2007; 120:682–691.
Kalies K-U, Allan S, Sergeyenko T, Kröger H, Römisch K . The protein translocation channel binds proteasomes to the endoplasmic reticulum membrane. EMBO J 2005; 24:2284–2293.
Kaiser ML, Römisch K . Proteasome 19S RP binding to the Sec61 channel plays a key role in ERAD. PLoS One 2015; 10:1–19.
Azzu V, Brand MD . Degradation of an intramitochondrial protein by the cytosolic proteasome. J Cell Sci 2010; 123:578–585.
Chan NC, Salazar AM, Pham AH, et al. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet 2011; 20:1726–1737.
Nakagawa T, Shirane M, Lemura SI, Natsume T, Nakayama KI . Anchoring of the 26S proteasome to the organellar membrane by FKBP38. Genes to Cells 2007; 12:709–719.
Sun XM, Butterworth M, MacFarlane M, Dubiel W, Ciechanover A, Cohen GM . Caspase activation inhibits proteasome function during apoptosis. Mol Cell 2004; 14:81–93.
Adrain C, Creagh EM, Cullen SP, Martin SJ . Caspase-dependent inactivation of proteasome function during programmed cell death in Drosophila and man. J Biol Chem 2004; 279:36923–36930.
Cuervo AM, Palmer A, Rivett AJ, Knecht E . Degradation of proteasomes by lysosomes in rat liver. Eur J Biochem 1995; 227:792–800.
Waite KA, De-La Mota-Peynado A, Vontz G, Roelofs J . Starvation induces proteasome autophagy with different pathways for core and regulatory particle. J Biol Chem 2015; 291:3239–3253.
Acknowledgements
Research in the laboratory of AC is supported by grants from the Dr Miriam and Sheldon G Adelson Medical Research Foundation (AMRF), the Israel Science Foundation (ISF), the I-CORE. Program of the Planning and Budgeting Committee and the ISF (Grant1775/12), and the DeutschIsraelische Projektkooperation (DIP). IL is supported by the Foulkes Fellowship. AC is an Israel Cancer Research Fund (ICRF) USA Professor.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 Unported License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Livneh, I., Cohen-Kaplan, V., Cohen-Rosenzweig, C. et al. The life cycle of the 26S proteasome: from birth, through regulation and function, and onto its death. Cell Res 26, 869–885 (2016). https://doi.org/10.1038/cr.2016.86
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2016.86