Abstract
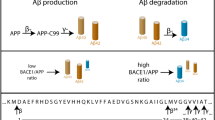
Mutations in the gene encoding the amyloid protein precursor (APP) cause autosomal dominant Alzheimer's disease1,2,3. Cleavage of APP by unidentified proteases, referred to as β- and γ-secretases4,5,6,7, generates the amyloid β-peptide, the main component of the amyloid plaques found in Alzheimer's disease patients8. The disease-causing mutations flank the protease cleavage sites in APP and facilitate its cleavage. Here we identify a new membrane-bound aspartyl protease (Asp2) with β-secretase activity. The Asp2 gene is expressed widely in brain and other tissues. Decreasing the expression of Asp2 in cells reduces amyloid β-peptide production and blocks the accumulation of the carboxy-terminal APP fragment that is created by β-secretase cleavage. Solubilized Asp2 protein cleaves a synthetic APP peptide substrate at the β-secretase site, and the rate of cleavage is increased tenfold by a mutation associated with early-onset Alzheimer's disease in Sweden3. Thus, Asp2 is a new protein target for drugs that are designed to block the production of amyloid β-peptide peptide and the consequent formation of amyloid plaque in Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Goate,A. et al. Segregation of a missense mutation in the amyloid protein precursor protein gene with familial Alzheimer's disease. Nature 349, 704–705 (1991).
Murrell,J., Farlow,M., Ghetti,B. & Benson,M. D. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science 254, 97–99 (1991).
Mullan,M. et al. A pathogenic mutation for probably Alzheimer's disease in the APP gene at the N-terminus of beta amyloid. Nature Genet. 1, 345–347 (1992).
Cai,X. D., Golde,T. E. & Younkin,S. G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science 259, 514–516 (1993).
Citron,M. et al. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature 360, 372–374 (1992).
Suzuki,N. et al. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science 264, 1336–1340 (1994).
Selkoe,D. J. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol. 8, 447–453 (1998).
Glenner,G. G. & Wong,C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloidogenic derivative. Science 255, 728–730 (1984).
Ladror,U. S., Snyder,S. W., Wang,G. T., Holzman,T. F. & Krafft,G. A. Cleavage at the amino and carboxyl termini of Alzheimer's amyloid-beta by cathepsin D. J. Biol. Chem. 269, 18422–18428 (1994).
Dreyer,R. N. et al. Processing of the pre-beta-amyloid protein by cathepsin D is enhanced by a familial Alzheimer's disease mutation. Eur. J. Biochem. 224, 265–271 (1994).
Saftig,P. et al. Amyloidogenic processing of human amyloid precursor protein in hippocampal neurons devoid of cathepsin D. J. Biol. Chem. 271, 27241–27244 (1996).
Tatnell, P. J. et al. Napsins: new human aspartic proteinases. Distinction between two closely related genes. FEBS Lett. 441, 43–48 (1994).
Neill,D., Hughes,D., Edwardson,B. K., Rima,B. K. & Allsop,D. Human IMR-32 neuroblastma cells as a model cell line in Alzheimer's Disease research. J. NeuroSci. Res. 39, 482–493 (1994).
Asami-Odaka,A., Ishibashi,Y., Kikuchi,T., Kitada,C. & Suzuki,N. Long amyloid β-protein secreted from wild-type human neuroblastoma IMR-32 cells. Biochemistry 34, 10272–10278 (1995).
De Strooper,B. et al. Deficiency of presenilin 1 inhibits cleavage of amyloid precursor protein. Nature 391, 387–390 (1998).
Naruse,S. et al. Effects of PS1 deficiency on membrane protein trafficking in neurons. Neuron 21, 1213–1221 (1998).
Vassar,R. et al. β-Secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741 (1999).
Hussain,I. et al. Identification of a novel aspartic protease (Asp2) as β-secretase. Molec. Cell. Neurosci. [online] 〈http://www.apnet.com/www/journal/cn/mcne.1999.0811〉
Pirttila,T. et al. Longitudinal study of cerebrospinal fluid amyloid proteins and apoliproprotein E in patients with probably Alzheimer's disease. Neurosci. Lett. 249, 21–24 (1998).
Acknowledgements
We thank C. Himes, M. Fairbanks, J. Leone, T. Emmons, R. Drong, J. Slightom, G. Winterrowd and D. McKinley for their help, and J. McCall for his unflagging support and good humour.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yan, R., Bienkowski, M., Shuck, M. et al. Membrane-anchored aspartyl protease with Alzheimer's disease β-secretase activity. Nature 402, 533–537 (1999). https://doi.org/10.1038/990107
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/990107
This article is cited by
-
The Alzheimer’s disease-linked protease BACE1 modulates neuronal IL-6 signaling through shedding of the receptor gp130
Molecular Neurodegeneration (2023)
-
Amyloid β-based therapy for Alzheimer’s disease: challenges, successes and future
Signal Transduction and Targeted Therapy (2023)
-
BACE1 in PV interneuron tunes hippocampal CA1 local circuits and resets priming of fear memory extinction
Molecular Psychiatry (2023)
-
The US9-Derived Protein gPTB9TM Modulates APP Processing Without Targeting Secretase Activities
Molecular Neurobiology (2023)
-
The metabolism of human soluble amyloid precursor protein isoforms is quantifiable by a stable isotope labeling-tandem mass spectrometry method
Scientific Reports (2022)