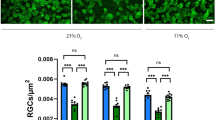
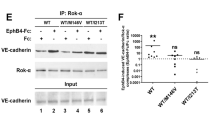
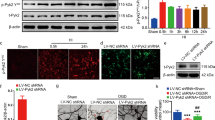
Abstract
Hypoxia stimulates angiogenesis through the binding of hypoxia-inducible factors to the hypoxia-response element in the vascular endothelial growth factor (Vegf) promotor. Here, we report that deletion of the hypoxia-response element in the Vegf promotor reduced hypoxic Vegf expression in the spinal cord and caused adult-onset progressive motor neuron degeneration, reminiscent of amyotrophic lateral sclerosis. The neurodegeneration seemed to be due to reduced neural vascular perfusion. In addition, Vegf165 promoted survival of motor neurons during hypoxia through binding to Vegf receptor 2 and neuropilin 1. Acute ischemia is known to cause nonselective neuronal death. Our results indicate that chronic vascular insufficiency and, possibly, insufficient Vegf-dependent neuroprotection lead to the select degeneration of motor neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nature Med. 6, 389–395 (2000).
Ferrara, N. & Alitalo, K. Clinical applications of angiogenic growth factors and their inhibitors. Nature Med. 5, 1359–1364 (1999).
Miao, H.Q. et al. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J. Cell. Biol. 146, 233–242 (1999).
Soker, S., Takashima, S., Miao, H.Q., Neufeld, G. & Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform- specific receptor for vascular endothelial growth factor. Cell 92, 735–745 (1998).
van Bruggen, N. et al. Vegf antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J. Clin. Invest. 104, 1613–1620 (1999).
Hayashi, T. et al. Expression of angiogenic factors in rabbit spinal cord after transient ischaemia. Neuropathol. Appl. Neurobiol. 25, 63–71 (1999).
Schratzberger, P. et al. Favorable effect of Vegf gene transfer on ischemic peripheral neuropathy. Nature Med. 6, 405–413 (2000).
Sondell, M., Lundborg, G. & Kanje, M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J. Neurosci. 19, 5731–5740 (1999).
Jin, K.L., Mao, X.O. & Greenberg, D.A. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc. Natl. Acad. Sci. USA 97, 10242–10247 (2000).
Dor, Y. & Keshet, E. Ischemia-driven angiogenesis. Trends Cardiovasc. Med. 7, 289–294 (1997).
Semenza, G.L. Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochem. Pharmacol. 59, 47–53 (2000).
Carmeliet, P. et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435–439 (1996).
Schultz, A. et al. Interindividual heterogeneity in the hypoxic regulation of VEGF: significance for the development of the coronary artery collateral circulation. Circulation 100, 547–552 (1999).
Bromberg, M.B. Pathogenesis of amyotrophic lateral sclerosis: a critical review. Curr. Opin. Neurol. 12, 581–588 (1999).
Green, S.L. & Tolwani, R.J. Animal models for motor neuron disease. Lab. Anim. Sci. 49, 480–487 (1999).
Robberecht, W.L. & de Jong, J.M.B.V. in Amyotrophic Lateral Sclerosis (eds. Brown, R.H.J., Meininger, V. & Swash, M.) 211–222 (Martin Dunitz, London, 2000).
Beal, M.F. et al. Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann. Neurol. 42, 644–654 (1997).
Ferrante, R.J. et al. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J. Neurochem. 69, 2064–2074 (1997).
Marti, H.H. & Risau, W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc. Natl. Acad. Sci. USA 95, 15809–15814 (1998).
Gupta, M., Mungai, P.T. & Goldwasser, E. A new transacting factor that modulates hypoxia-induced expression of the erythropoietin gene. Blood 96, 491–497 (2000).
Stein, I. et al. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol. Cell. Biol. 18, 3112–3119 (1998).
Levy, N.S., Chung, S., Furneaux, H. & Levy, A.P. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J. Biol. Chem. 273, 6417–6423 (1998).
Damert, A., Ikeda, E. & Risau, W. Activator-protein-1 binding potentiates the hypoxia-induciblefactor-1- mediated hypoxia-induced transcriptional activation of vascular- endothelial growth factor expression in C6 glioma cells. Biochem. J. 327, 419–423 (1997).
Dal Canto, M.C. & Gurney, M.E. Development of central nervous system pathology in a murine transgenic model of human amyotrophic lateral sclerosis. Am. J. Pathol. 145, 1271–1279 (1994).
Lee, M.K. & Cleveland, D.W. Neuronal intermediate filaments. Annu. Rev. Neurosci. 19, 187–217 (1996).
Martin, L.J., Price, A.C., Kaiser, A., Shaikh, A.Y. & Liu, Z. Mechanisms for neuronal degeneration in amyotrophic lateral sclerosis and in models of motor neuron death. Int. J. Mol. Med. 5, 3–13 (2000).
Kawamura, Y., Okazaki, H., O'Brien, P.C. & Dych, P.J. Lumbar motoneurons of man: I) number and diameter histogram of alpha and gamma axons of ventral root. J. Neuropathol. Exp. Neurol. 36, 853–360 (1977).
Cashman, N.R. et al. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev. Dyn. 194, 209–221 (1992).
Kong, J. & Xu, Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J. Neurosci. 18, 3241–3250 (1998).
Theys, P.A., Peeters, E. & Robberecht, W. Evolution of motor and sensory deficits in amyotrophic lateral sclerosis estimated by neurophysiological techniques. J. Neurol. 246, 438–442 (1999).
Kostic, V. et al. Midbrain dopaminergic neuronal degeneration in a transgenic mouse model of familial amyotrophic lateral sclerosis. Ann. Neurol. 41, 497–504 (1997).
Williamson, T.L. et al. Absence of neurofilaments reduces the selective vulnerability of motor neurons and slows disease caused by a familial amyotrophic lateral sclerosis-linked superoxide dismutase 1 mutant. Proc. Natl. Acad. Sci. USA 95, 9631–9636 (1998).
Ogunshola, O.O. et al. Neuronal Vegf expression correlates with angiogenesis in postnatal developing rat brain. Brain Res. Dev. Brain Res. 119, 139–153 (2000).
Lang-Lazdunski, L. et al. Spinal cord ischemia. Development of a model in the mouse. Stroke 31, 208–213 (2000).
Nohl, H., Staniek, K. & Gille, L. Imbalance of oxygen activation and energy metabolism as a consequence or mediator of aging. Exp. Gerontol. 32, 485–500 (1997).
Ochiai-Kanai, R., Hasegawa, K., Takeuchi, Y., Yoshioka, H. & Sawada, T. Immunohistochemical nitrotyrosine distribution in neonatal rat cerebrocortical slices during and after hypoxia. Brain Res. 847, 59–70 (1999).
Knight, J.A. Reactive oxygen species and the neurodegenerative disorders. Ann. Clin. Lab. Sci. 27, 11–25 (1997).
Rivard, A. et al. Age-dependent defect in VEGF expression is associated with reduced HIF1a activity. J. Biol. Chem. 275, 29643–29647 (2000).
Hellwig-Burgel, T., Rutkowski, K., Metzen, E., Fandrey, J. & Jelkmann, W. Interleukin-1beta and tumor necrosis factor-alpha stimulate DNA binding of hypoxia-inducible factor-1. Blood 94, 1561–1567 (1999).
He, Z. & Tessier-Lavigne, M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 90, 739–751 (1997).
Yu, H.H. & Kolodkin, A.L. Semaphorin signaling: a little less per-plexin. Neuron 22, 11–14 (1999).
Raper, J.A. Semaphorins and their receptors in vertebrates and invertebrates. Curr. Opin. Neurobiol. 10, 88–94 (2000).
Plate, K.H., Beck, H., Danner, S., Allegrini, P.R. & Wiessner, C. Cell type specific upregulation of vascular endothelial growth factor in an MCA-occlusion model of cerebral infarct. J. Neuropathol. Exp. Neurol. 58, 654–666 (1999).
Carmeliet, P. et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nature Med. 5, 495–502 (1999).
Gorselink, M. et al. Accurate measurements of in situ isometric contractile properties of hind limb plantar and dorsal flexor muscle complex of intact mice. Eur. J. Physiol. 439, 665–670 (2000).
Vandenberghe, W., Van Den Bosch, L. & Robberecht, W. Glial cells potentiate kainate-induced neuronal death in a motoneuron- enriched spinal coculture system. Brain Res. 807, 1–10 (1998).
Acknowledgements
We thank F. Bono (Synthélabo), S. Thom (Erlangen), A. Westmuckett and D. Goulding (London), and K. Bijnens, A. Bouché, S. De Cat, M. De Mol, I. Cartois, K. De Roover, E. Gils, B. Hermans, S. Jansen, L. Kieckens, Y.W. Man, A. Manderveld, K. Maris, A. Sahli, T. Vancoetsem, A. Vandenhoeck, P. Vanwesemael, B. Vanwetswinkel and S. Wyns (CTG, Belgium) for assistance. This work was supported by the European Community (Biomed BMH4-CT98-3380), Actie Levenslijn (#7.0019.98), FWO (G012500) and the Schwerpunktprogramm 1069 Angiogenese (GFG Pl 158-4/1). G.T. is a postdoctoral fellow of the DFG (Germany) B.O. is a fellow of the IWT, and L.V.D.B. is a postdoctoral fellow and W.R. is a clinical investigator of the FWO. This work was supported by GOA/93/03.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oosthuyse, B., Moons, L., Storkebaum, E. et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet 28, 131–138 (2001). https://doi.org/10.1038/88842
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/88842
This article is cited by
-
Biology and therapeutic targeting of vascular endothelial growth factor A
Nature Reviews Molecular Cell Biology (2023)
-
Targeting angiogenesis in oncology, ophthalmology and beyond
Nature Reviews Drug Discovery (2023)
-
Preservation of KCC2 expression in axotomized abducens motoneurons and its enhancement by VEGF
Brain Structure and Function (2023)
-
Granulocyte-colony stimulating factor (G-CSF): an emerging therapeutic approach for amyotrophic lateral sclerosis (ALS)
Acta Neurologica Belgica (2023)
-
Can the administration of platelet lysates to the brain help treat neurological disorders?
Cellular and Molecular Life Sciences (2022)