Abstract
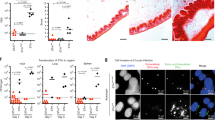
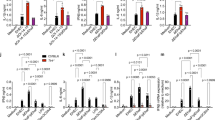
Antibacterial peptides are active defense components of innate immunity. Several studies confirm their importance at epithelial surfaces as immediate barrier effectors in preventing infection. Here we report that early in Shigella spp. infections, expression of the antibacterial peptides LL-37 and human β-defensin-1 is reduced or turned off. The downregulation is detected in biopsies from patients with bacillary dysenteries and in Shigella- infected cell cultures of epithelial and monocyte origin. This downregulation of immediate defense effectors might promote bacterial adherence and invasion into host epithelium and could be an important virulence parameter. Analyses of bacterial molecules causing the downregulation indicate Shigella plasmid DNA as one mediator.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Frohm, M. et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272, 15258–15263 (1997).
Stolzenberg, E.D., Anderson, G.M., Ackermann, M.R., Whitlock, R.H. & Zasloff, M. Epithelial antibiotic induced in states of disease. Proc. Natl. Acad. Sci. USA 94, 8686–8690 (1997).
Tarver, A.P. et al. Enteric β-defensin: molecular cloning and characterization of a gene with inducible intestinal epithelial cell expression associated with Cryptosporidium parvum infection. Infect. Immun. 66, 1045–1056 (1998).
Bals, R., Wang, X., Zasloff, M. & Wilson, J.M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 95, 9541–9546 (1998).
Agerberth, B. et al. Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am. J. Respir. Crit. Care Med. 160, 283–290 (1999).
Zhao, C., Wang, I. & Lehrer, R.I. Widespread expression of β-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 396, 319–322 (1996).
Harder, J., Bartels, J., Christophers, E. & Schroder, J.M. A peptide antibiotic from human skin. Nature 387, 861 (1997).
Jones, D.E. & Bevins, C.L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J. Biol. Chem. 267, 23216–23225 (1992).
Jones, D.E. & Bevins, C.L. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 315, 187–192 (1993).
Bevins, C.L., Martin-Porter, E. & Ganz, T. Defensins and innate host defence of the gastrointestinal tract. Gut 45, 911–915 (1999).
Boman, H.G. Antibacterial peptides: key components needed in immunity. Cell 65, 205–207 (1991).
O'Neil, D.A. et al. Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 163, 6718–6724 (1999).
O'Neil, D.A. et al. Regulation of human β-defensins by gastric epithelial cells in response to infection with helicobacter pylori or stimulation with interleukin-1. Infect. Immun. 68, 5412–5415 (2000).
Dorman, C.J. & Porter, M.E. The Shigella virulence gene regulatory cascade: a paradigm of bacterial gene control mechanisms. Mol. Microbiol. 29, 677–684 (1998).
Menard, R., Dehio, C. & Sansonetti, P.J. Bacterial entry into epithelial cells: the paradigm of Shigella. Trends Microbiol. 4, 220–226 (1996).
Zychlinsky, A. & Sansonetti, P.J. Apoptosis as a proinflammatory event: what can we learn from bacteria- induced cell death? Trends Microbiol. 5, 201–204 (1997).
Sorensen, O., Cowland, J.B., Askaa, J. & Borregaard, N. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J. Immunol. Methods 206, 53–59 (1997).
Sansonetti, P.J. Molecular and cellular mechanisms of invasion of the intestinal barrier by enteric pathogens. The paradigm of Shigella. Folia Microbiol. 43, 239–246 (1998).
Agerberth, B. et al. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood 96, 3086–3093 (2000).
Kagnoff, M.F. & Eckmann, L. Epithelial cells as sensors for microbial infection. J. Clin. Invest. 100, 6–10 (1997).
Bliska, J.B., Galan, J.E. & Falkow, S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell 73, 903–920 (1993).
Siebers, A. & Finlay, B.B. Models to study enteropathogenic bacteria: lessons learned from Shigella. Trends Microbiol. 3, 207–209 (1995).
Albert, M.J., Faruque, A.S., Faruque, S.M., Sack, R.B. & Mahalanabis, D. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J. Clin. Microbiol. 37, 3458–3464 (1999).
Islam, D., Wretlind, B., Ryd, M., Lindberg, A.A. & Christensson, B. Immunoglobulin subclass distribution and dynamics of Shigella-specific antibody responses in serum and stool samples in shigellosis. Infect. Immun. 63, 2054–2061 (1995).
Raqib, R. et al. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect. Immun. 63, 289–296 (1995).
Islam, D., Veress, B., Bardhan, P.K., Lindberg, A.A. & Christensson, B. In situ characterization of inflammatory responses in the rectal mucosae of patients with shigellosis. Infect. Immun. 65, 739–749 (1997).
Islam, D., Veress, B., Bardhan, P.K., Lindberg, A.A. & Christensson, B. Quantitative assessment of IgG and IgA subclass producing cells in rectal mucosa during shigellosis. J. Clin. Pathol. 50, 513–520 (1997).
Mandic-Mulec, I., Weiss, J. & Zychlinsky, A. Shigella flexneri is trapped in polymorphonuclear leukocyte vacuoles and efficiently killed. Infect. Immun. 65, 110–115 (1997).
Shafer, W.M., Qu, X., Waring, A.J. & Lehrer, R.I. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. USA 95, 1829–1833 (1998).
Guo, L. et al. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95, 189–198 (1998).
Krieg, A.M. The role of CpG motifs in innate immunity. Curr. Opin. Immunol. 12, 35–43 (2000).
Wagner, H. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv. Immunol. 73, 329–368 (1999).
Chu, R.S. et al. CpG oligodeoxynucleotides downregulate macrophage class II MHC antigen processing. J. Immunol. 163, 1188–1194 (1999).
Gudmundsson, G.H. et al. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 238, 325–332 (1996).
Larrick, J.W. et al. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 63, 1291–1297 (1995).
Cowland, J.B., Johnsen, A.H. & Borregaard, N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 368, 173–176 (1995).
Acknowledgements
We thank the participants in the study; N.H. Alam for help with biopsy samples; G. Ara and Sharifunnahar for technical assistance; Masuda and Momtaz for assistance in recruiting patients and control subjects; E. Ólafsson for help with statistical analysis; K. Nilsson for the gift of the U937 cell-line; R.A. Harris for linguistic advice; and B. Axelsson for technical assistance. This study was supported by grants from Swedish Agency for Research Co-operation with Developing Countries (SAREC), Centre for Diarrhoeal Disease Research, Bangladesh, the Swedish Medical Research Council, The Swedish Foundation for Strategic Research, the Swedish Cancer Society, Magnus Bergvall's Foundation, Åke Wiberg's Foundation, Ruth and Richard Julin's Foundation and Prof. Nanna Svartz' Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Islam, D., Bandholtz, L., Nilsson, J. et al. Downregulation of bactericidal peptides in enteric infections: A novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med 7, 180–185 (2001). https://doi.org/10.1038/84627
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/84627