Abstract
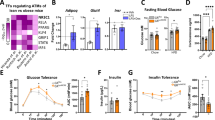
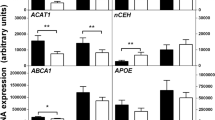
Peroxisome proliferator-activated receptor-γ (PPAR-γ), the transcription factor target of the anti-diabetic thiazolidinedione (TZD) drugs, is reported to mediate macrophage differentiation and inflammatory responses. Using PPAR-γ–deficient stem cells, we demonstrate that PPAR-γ is neither essential for myeloid development, nor for such mature macrophage functions as phagocytosis and inflammatory cytokine production. PPAR-γ is required for basal expression of CD36, but not for expression of the other major scavenger receptor responsible for uptake of modified lipoproteins, SR-A. In wild-type macrophages, TZD treatment divergently regulated CD36 and class A macrophage-scavenger receptor expression and failed to induce significant cellular cholesterol accumulation, indicating that TZDs may not exacerbate macrophage foam-cell formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kliewer, S.A., Umesono, K., Noonan, D.J., Heyman, R.A. & Evans, R.M. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature 358, 771–774 (1992).
Kliewer, S.A., Umesono, K., Mangelsdorf, D.J. & Evans, R.M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature 355, 446–449 (1992).
Spiegelman, B.M. Peroxisome proliferator-activated receptor γ: A key regulator of adipogenesis and systemic insulin sensitivity. Eur. J. Med. Res. 2, 457–464 (1997).
Law, R.E. et al. Expression and function of PPAR-γ in rat and human vascular smooth muscle cells. Circulation 101, 1311–1318 (2000).
Clark, R.B. et al. The nuclear receptor PPAR-γ and immunoregulation: PPAR-γ mediates inhibition of helper T cell responses. J. Immunol. 164, 1364–1371 (2000).
Forman, B.M. et al. 15-Deoxy-Δ12,14prostaglandin J2 is a ligand for the adipocyte determination factor PPAR-γ. Cell 83, 803–812 (1995).
Lehmann, J.M. et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPAR-γ). J. Biol. Chem. 270, 12953–12956 (1995).
Kliewer, S.A. et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc. Natl. Acad. Sci. USA 94, 4318–4323 (1997).
Tontonoz, P., Nagy, L., Alvarez, J.G.A., Thomazy, V.A. & Evans, R. PPAR-γ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93, 241–252 (1998).
Nagy, L., Tontonoz, P., Alvarez, J.G.A., Chen, H. & Evans, R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPAR-γ. Cell 93, 229–240 (1998).
Ricote, M., Li, A.C., Willson, T.M., Kelly, C.J. & Glass, C.K. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature 391, 79–82 (1998).
Jiang, C., Ting, A.T. & Seed, B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 391, 82–86 (1998).
Marx, N., Sukhova, G., Murphy, C., Libby, P. & Plutzky, J. Macrophages in human atheroma contain PPAR-γ: differentiation-dependent peroxisomal proliferator-activated receptor γ (PPAR-γ) expression and reduction of MMP-9 activity through PPAR-γ activation in mononuclear phagocytes in vitro. Am. J. Pathol. 153, 17–23 (1998).
Ricote, M. et al. Expression of the peroxisome proliferator-activated receptor γ (PPAR-γ) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoproteins. Proc. Natl. Acad. Sci. USA 95, 7614–7619 (1998).
Chinetti, G. et al. CLA-1/SR-BI is expressed in atherosclerotic lesion macrophages and regulated by activators of peroxisome proliferator-activated receptors. Circulation 101, 2411–2417 (2000).
Kersten, S., Desvergne, B. & Wahli, W. Roles of PPARs in health and disease. Nature 405, 421–424 (2000).
Willoughby, D.A., Moore, A.R. & Colville-Nash, P.R. Cyclopentenone prostaglandins—new allies in the war on inflammation [news]. Nature Med. 6, 137–138 (2000).
Rossi, A. et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature 403, 103–108 (2000).
Straus, D.S. et al. 15-Deoxy-Δ12,14prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway [In Process Citation]. Proc. Natl. Acad. Sci. US A 97, 4844–4849 (2000).
Vaidya, S., Somers, E.P., Wright, S.D., Detmers, P.A. & Bansal, V.S. 15-Deoxy-Δ12,14prostaglandin J2 inhibits the β-2 integrin-dependent oxidative burst: involvement of a mechanism distinct from peroxisome proliferator-activated receptor γ ligation. J. Immunol. 163, 6187–6192 (1999).
Pasceri, V., Wu, H.D., Willerson, J.T. & Yeh, E.T. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-γ activators. Circulation 101, 235–238 (2000).
Spiegelman, B.M. PPAR-γ in monocytes: less pain, any gain? Cell 93, 153–155 (1998).
Barak, Y. et al. PPAR-γ is required for placental, cardiac, and adipose tissue development. Mol. Cell 4, 585–595 (1999).
Rosen, E.D. et al. PPAR-γ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4, 611–617 (1999).
Kubota, N. et al. PPAR-γ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell 4, 597–609 (1999).
Moore, K.J., Fabunmi, R.P., Andersson, L.P. & Freeman, M.W. In vitro-differentiated embryonic stem cell macrophages: a model system for studying atherosclerosis-associated macrophage functions. Arterio. Thromb. Vasc. Biol. 18, 1647–1654 (1998).
Thieringer, R. et al. Activation of peroxisome proliferator-activated receptor γ does not inhibit IL-6 or TNF-α responses of macrophages to lipopolysaccharide in vitro or in vivo. J. Immunol. 164, 1046–1054 (2000).
Huang, J.T. et al. Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15-lipoxygenase. Nature 400, 378–382 (1999).
Feng, J. et al. Induction of CD36 expression by oxidized LDL and IL-4 by a common signaling pathway dependent on protein kinase C and PPAR-γ. J. Lipid Res. 41, 688–696 (2000).
de Villiers, W.J., Fraser, I.P., Hughes, D.A., Doyle, A.G. & Gordon, S. Macrophage-colony-stimulating factor selectively enhances macrophage scavenger receptor expression and function. J. Exp. Med. 180, 705–709 (1994).
Armesilla, A.L., Calvo, D. & Vega, M.A. Structural and functional characterization of the human CD36 gene promoter: identification of a proximal PEBP2/CBF site. J. Biol. Chem. 271, 7781–7787 (1996).
Febbraio, M. et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J. Biol. Chem. 274, 19055–19062 (1999).
Febbraio, M. et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 105, 1049–1056 (2000).
Calvo, D., Gomez-Coronado, D., Suarez, Y., Lasuncion, M.A. & Vega, M.A. Human CD36 is a high affinity receptor for the native lipoproteins HDL, LDL, and VLDL. J. Lipid Res. 39, 777–788 (1998).
Huh, H.Y., Pearce, S.F., Yesner, L.M., Schindler, J.L. & Silverstein, R.L. Regulated expression of CD36 during monocyte-to-macrophage differentiation: potential role of CD36 in foam cell formation. Blood 87, 2020–2028 (1996).
Repa, J.J. et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers [see comments]. Science 289, 1524–1529 (2000).
Li, A.C. et al. Peroxisome proliferator-activated receptor γ ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 106, 523–531 (2000).
Ghazzi, M.N. et al. Cardiac and glycemic benefits of troglitazone treatment in NIDDM. The Troglitazone Study Group. Diabetes 46, 433–439 (1997).
Noguchi, N. et al. Inhibition of oxidation of low density lipoprotein by troglitazone. Atherosclerosis 123, 227–234 (1996).
Minamikawa, J., Yamauchi, M., Inoue, D. & Koshiyama, H. Another potential use of troglitazone in noninsulin-dependent diabetes mellitus [letter; comment]. J. Clin. Endocrinol. Metab. 83, 1041–1042 (1998).
Law, R.E. et al. Troglitazone inhibits vascular smooth muscle cell growth and intimal hyperplasia. J. Clin. Invest. 98, 1897–1905 (1996).
Shinohara, E. et al. Troglitazone suppresses intimal formation following balloon injury in insulin-resistant Zucker fatty rats. Atherosclerosis 136, 275–279 (1998).
Freeman, M. et al. An ancient, highly conserved family of cysteine-rich protein domains revealed by cloning type I and type II murine macrophage scavenger receptors. Proc. Natl. Acad. Sci. USA 87, 8810–8814 (1990).
Milstone, D.S., Bradwin, G. & Mortensen, R.M. Simultaneous Cre catalyzed recombination of two alleles to restore neomycin sensitivity and facilitate homozygous mutations. Nucleic Acids Res. 27, 10 (1999).
Lindblad-Toh, K. et al. Large-scale discovery and genotyping of single-nucleotide polymorphisms in the mouse. Nature Genet. 24, 381–386 (2000).
Jacobs, N.L. et al. Analysis of a Chinese hamster ovary cell mutant with defective mobilization of cholesterol from the plasma membrane to the endoplasmic reticulum. J. Lipid Res. 38, 1973–1987 (1997).
Ashkenas, J. et al. Structures and high and low affinity ligand binding properties of murine type I and type II macrophage scavenger receptors. J. Lipid. Res. 34, 983–1000 (1993).
Acknowledgements
We thank L. Liscum for help in performing the cellular cholesterol measurements by gas chromatography; L. Shang and R. Lane for their technical assistance; and B. Seed and H. Kronenberg for comments. This work was supported by grants from the NHLBI [(45098 (MWF), 56985 (MWF), HL5409 (DSM)] and NIDDK [50305 (MWF)].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moore, K., Rosen, E., Fitzgerald, M. et al. The role of PPAR-γ in macrophage differentiation and cholesterol uptake. Nat Med 7, 41–47 (2001). https://doi.org/10.1038/83328
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/83328
This article is cited by
-
Association study of rs1801282 PPARG gene polymorphism and immune cells and cytokine levels in a Spanish pregnant women cohort and their offspring
Journal of Biomedical Science (2020)
-
Molecular determinants for the polarization of macrophage and osteoclast
Seminars in Immunopathology (2019)
-
Statin as a novel pharmacotherapy of pulmonary alveolar proteinosis
Nature Communications (2018)
-
Antioxidants in the Fight Against Atherosclerosis: Is This a Dead End?
Current Atherosclerosis Reports (2018)
-
Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation
Cellular and Molecular Neurobiology (2018)