Abstract
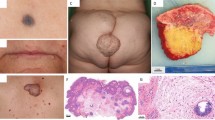
Carney complex (CNC) is a multiple neoplasia syndrome characterized by spotty skin pigmentation, cardiac and other myxomas, endocrine tumours and psammomatous melanotic schwannomas1,2,3,4,5. CNC is inherited as an autosomal dominant trait and the genes responsible have been mapped to 2p16 and 17q22–24 (refs 6, 7). Because of its similarities to the McCune-Albright syndrome5,8 and other features, such as paradoxical responses to endocrine signals9, genes implicated in cyclic nucleotide-dependent signalling have been considered candidates for causing CNC (ref. 10). In CNC families mapping to 17q, we detected loss of heterozygosity (LOH) in the vicinity of the gene (PRKAR1A) encoding protein kinase A regulatory subunit 1-α (RIα), including a polymorphic site within its 5′ region. We subsequently identified three unrelated kindreds with an identical mutation in the coding region of PRKAR1A. Analysis of additional cases revealed the same mutation in a sporadic case of CNC, and different mutations in three other families, including one with isolated inherited cardiac myxomas. Analysis of PKA activity in CNC tumours demonstrated a decreased basal activity, but an increase in cAMP-stimulated activity compared with non-CNC tumours. We conclude that germline mutations in PRKAR1A, an apparent tumour-suppressor gene, are responsible for the CNC phenotype in a subset of patients with this disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Carney, J.A. & Young, W.F. Primary pigmented nodular adrenocortical disease and its associated conditions. Endocrinologist 2, 6–21 (1992).
Carney, J.A. Carney complex: the complex of myxomas, spotty pigmentation, endocrine overactivity, and schwannomas. Semin. Dermatol. 14, 90 –98 (1995).
Stratakis, C.A., Kirschner, L.S. & Carney, J.A. Carney complex: diagnosis and management of the complex of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas . Am. J. Med. Genet. 80, 183– 185 (1998).
Carney, J.A., Hruska, L.S., Beauchamp, G.D. & Gordon, H. Dominant inheritance of the complex of myxomas, spotty pigmentation and endocrine overactivity. Mayo Clin. Proc. 61, 165– 172 (1985).
Stratakis, C.A. Genetics of Carney complex and related familial lentiginoses, and other multiple tumor syndromes. Front. Biosci. 5, D353– D366 (2000).
Stratakis, C.A. et al. Carney complex, a familial multiple neoplasia and lentiginosis syndrome: analysis of 11 kindreds and linkage to the short arm of chromosome 2. J. Clin. Invest. 97, 699– 705 (1996).
Casey, M. et al. Identification of a novel genetic locus for familial cardiac myxomas and Carney complex. Circulation 98, 2560–2566 (1998).
Weinstein, L.S. et al. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N. Engl. J. Med. 325, 1688– 1695 (1991).
Stratakis, C.A. et al. Paradoxical response to dexamethasone in the diagnosis of primary pigmented nodular adrenocortical disease. Ann. Intern. Med. 131, 585–591 ( 1999).
DeMarco, L. et al. Sporadic cardiac myxomas and tumors from patients with Carney complex are not associated with activating mutations of the Gsa gene. Hum. Genet. 98, 185–188 (1996).
Schoenberg-Fejzo, M. et al. Integrated map of chromosome 17q critical region in multiple sclerosis. Am. J. Hum. Genet. 65, A442 (1999).
Danoff, A., Jormark, S., Lorber, D. & Fleischer, N. Adrenocortical micronodular dysplasia, cardiac myxomas, lentigines and spindle cell tumors: report of a kindred. Arch. Intern. Med. 143, 443–448 (1987).
Solberg, R. et al. The human gene for the regulatory subunit RI a of cyclic adenosine 3′, 5′-monophosphate-dependent protein kinase: two distinct promoters provide differential regulation of alternately spliced messenger ribonucleic acids. Endocrinology 138, 169– 181 (1997).
Stratakis, C.A., Kirschner, L.S., Taymans, S.E., Carney, J.A. & Basson, C.T. Genetic heterogeneity in Carney Complex (OMIM 160980): Contributions of loci at chromosomes 2 and 17 in its genetics. Am. J. Hum. Genet. 65, A447 (1999).
Liebler, G.A. et al. Familial myxomas in four siblings. J. Thorac. Cardiovasc. Surg. 71, 605–608 ( 1976)
Cho, Y.S. et al. Extracellular protein kinase A as a cancer biomarker: its expression by tumor cells and reversal by a myristate-lacking Ca and RIIb subunit overexpression . Proc. Natl Acad. Sci. USA 97, 837– 840 (2000).
Sun, X., Perlick, H.A., Dietz, H.C. & Maquat, L.E. A mutated human homologue to yeast Upf1 protein has a dominant-negative effect on the decay of nonsense-containing mRNAs in mammalian cells. Proc. Natl Acad. Sci. USA 95, 10009– 10014 (1998).
Tautz, D. Hypervariability of simple sequences as a general source for polymorphic DNA markers. Nucleic Acids Res. 17, 6463– 6471 (1989).
Cummings, D.E. et al. Genetically lean mice result from targeted disruption of the RIIb subunit of protein kinase A. Nature 382, 622–626 (1996).
Amieux, P.S. et al. Compensatory regulation of RIa protein levels in protein kinase A mutant mice. J. Biol. Chem. 272, 3993– 3998 (1997).
Scott, J.D. Cyclic nucleotide-dependent protein kinases. Pharmacol. Ther. 50, 123–145 (1991).
Orellana, S.A. & McKnight, G.S. Mutations in the catalytic subunit of cAMP-dependent protein kinase result in unregulated biological activity. Proc. Natl Acad. Sci. USA 89, 4726–4730 (1992).
Beebe, S.J., Holloway, R., Rannels, S.R. & Corbin, J.D. Two classes of cAMP analogs which are selective for the two different cAMP-binding sites of type II protein kinase demonstrate synergism when added together to intact adipocytes. J. Biol. Chem. 259, 3539–3547 (1984).
Stratakis, C.A. et al. Cytogenetic and microsatellite alterations in tumors from patients with the syndrome of myxomas, spotty skin pigmentation, and endocrine overactivity (Carney complex). J. Clin. Endocrinol. Metab. 81, 3607–3614 (1996).
Stratakis, C.A. et al. Carney complex, Peutz-Jeghers syndrome, Cowden disease, and Bannayan-Zonana syndrome share cutaneous and endocrine manifestations, but not genetic loci. J. Clin. Endocrinol. Metab. 83, 2972–2976 (1998).
O'Donovan, M.C. et al. Blind analysis of denaturing high-performance liquid chromatogarphy as a tool for mutation detection. Genomics 52, 44–49 (1998).
Jones, A.C. et al. Optimal temperature selection for mutation detection by denaturing high performance liquid chromatography and comparison to SSCP and heteroduplex analysis. Clin. Chem. 45, 1133– 1140 (1999).
Kuo, J.F., Krueger, B.K., Sanes, J.R. & Greengard, P. Cyclic nucleotide-dependent protein kinases. V. Preparation and properties of adenosine 3′, 5′-monophosphate-dependent protein kinase from various bovine tissues. Biochem. Biophys. Acta 212, 79–91 (1970).
Acknowledgements
We thank the patients and their families for their support and patience; the physicians who have sent us patients and patient samples over the years, particularly R. Pyeritz, S. Mendoza, K. Tuckerman, J. Toppari and A. Reza; M. Schoenberg-Fejzo and L. Peltonen for discussions on the status of the chromosome 17 genetic and physical maps; S. Marx for discussions on multiple endocrine neoplasias; R. Alexander, S. Libutti, D. Burtlet and E. Oldfield for surgical services; C. Basson for collaboration on the first chromosome 17 linkage study; J. Monbo and L. Goldman for technical assistance; C. Bondy and I. Dawid for critical review of the data; and the nursing and other support staff on the 8W and 9W inpatient wards at the NIH for their support of our research studies and their help in the management of patients with Carney complex.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kirschner, L., Carney, J., Pack, S. et al. Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet 26, 89–92 (2000). https://doi.org/10.1038/79238
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/79238