Abstract
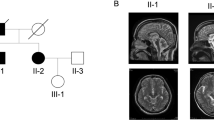
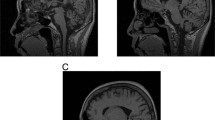
Myotonic dystrophy (DM) is the only disease reported to be caused by a CTG expansion. We now report that a non-coding CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). This expansion, located on chromosome 13q21, was isolated directly from the genomic DNA of an ataxia patient by RAPID cloning. SCA8 patients have expansions similar in size (107-127 CTG repeats) to those found among adult-onset DM patients. SCA8 is the first example of a dominant SCA not caused by a CAG expansion translated as a polyglutamine tract.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Subramony, S.H. & Currier, R.D. The classification of familial ataxias. in Handbook of Clinical Neurology (eds Vinken, P.J., Bruyn, G.W. & Klawans, H.L.) 271–284 (Elsevier Science Publishing Company, New York, 1991).
Harding, A.E. The clinical features and classification of the late onset autosomal dominant cerebellar ataxia: a study of 11 families, including descendants of 'The Drew Family of Walworth'. Brain 105, 1–28 (1982).
Zoghbi, H.Y. The spinocerebellar degenerations. Curr. Neurol. 11, 121–144 (1991).
Brice, A. Unstable mutations and neurodegenerative disorders. J. Neurol. 245, 505–510 (1998).
Klockgether, T. & Evert, B. Genes involved in hereditary ataxias. Trends Neurosci. 21, 413–418 (1998).
Jansen, G. et al. Abnormal myotonic dystrophy protein kinase levels produce only mild myopathy in mice. Nature Genet. 13, 316–324 (1996).
Reddy, S. et al. Mice lacking the myotonic dystrophy protein kinase develop a late onset progressive myopathy. Nature Genet. 13, 325–335 (1996).
Groenen, P. & Wieringa, B. Expanding complexity in myotonic dystrophy. Bioessays 20, 901–912 (1998).
Hoffmann-Radvanyi, H. et al. Myotonic dystrophy: absence of CTG enlarged transcript in congenital forms, and low expression of the normal allele. Hum. Mol. Genet. 2, 1263–1266 (1993).
Krahe, R. et al. Effect of myotonic dystrophy trinucleotide repeat expansion on DMPK transcription and processing. Genomics 28, 1–14 (1995).
Taneja, K.L., McCurrach, M., Schalling, M., Housman, D. & Singer, R.H. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J. Cell Biol. 128, 995–1002 (1995).
Davis, B.M., McCurrach, M.E., Taneja, K.L., Singer, R.H. & Housman, D.E. Expansion of a CUG trinucleotide repeat in the 3´ untranslated region of myotonic dystrophy protein kinase transcripts results in nuclear retention of transcripts. Proc. Natl Acad. Sci. USA 94, 7388–7393 (1997).
Timchenko, L.T. et al. Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucleic Acids Res. 24, 4407–4414 (1996).
Roberts, R. et al. Altered phosphorylation and intracellular distribution of a (CUG)n triplet repeat RNA-binding protein in patients with myotonic dystrophy and in myotonin protein kinase knockout mice. Proc. Natl Acad. Sci. USA 94, 13221–13226 (1997).
Philips, A.V., Timchenko, L.T. & Cooper, T.A. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science 280, 737–741 (1998).
Schalling, M., Hudson, T., Buetow, K. & Housman, D. Direct detection of novel expanded trinucleotide repeats in the human genome. Nature Genet. 4, 135–139 (1993).
Koob, M.D. et al. Rapid cloning of expanded trinucleotide repeat sequences from genomic DNA. Nature Genet. 18, 72–75 (1998).
Moseley, M.L. et al. Incidence of dominant spinocerebellar and Friedreich triplet repeats among 361 ataxia families. Neurology 51, 1666–1671 (1998).
Chung, M.-y. et al. Evidence for a mechanism predisposing to intergenerational CAG repeat instability in spinocerebellar ataxia type 1. Nature Genet. 5, 254–258 (1993).
Cancel, G. et al. Molecular and clinical correlations in spinocerebellar ataxia 2×a study of 32 families. Hum. Mol. Genet. 6, 709–715 (1997).
Maruyama, H. et al. Molecular features of the CAG repeats and clinical manifestation of Machado-Joseph disease. Hum. Mol. Genet. 4, 807–812 (1995).
Maciel, P. et al. Correlation between CAG repeat length and clinical features in Machado-Joseph disease. Am. J. Hum. Genet. 57, 54–61 (1995).
Zhuchenko, O. et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the ×-1A-voltage-dependent calcium channel. Nature Genet. 15, 62–69 (1997).
Jodice, C. et al. Episodic ataxia type 2 (EA2) and spinocerebellar atxia type 6 (SCA6) due to CAG repeat expansion in the CACNA1A gene on chromosome 19p. Hum. Mol. Genet. 6, 1973–1978 (1997).
David, G. et al. Molecular and clinical correlations in autosomal dominant cerebellar ataxia with progressive macular dystrophy (SCA7). Hum. Mol. Genet. 7, 165–170 (1998).
Tsilfidis, C., MacKenzie, A.E., Mettler, G., Barcelo, J. & Korneluk, R.G. Correlation between CTG trinucleotide repeat length and frequency of severe congenital myotonic dystrophy. Nature Genet. 1, 192–195 (1992).
Vanhee-Brossollet, C. & Vaquero, C. Do natural antisense transcripts make sense in eukaryotes? Gene 211, 1–9 (1998).
Nellen, W. & Lichtenstein, C. What makes an mRNA anti-sense-itive? Trends Biochem. Sci. 18, 419–423 (1993).
Lathrop, G.M., Lalouel, J.M., Julier, C. & Ott, J. Strategies for multilocus linkage analysis in humans. Proc. Natl Acad. Sci. USA 81, 3443–3446 (1984).
Acknowledgements
We thank family members for their participation, C. Peterson, H. Lipe and D. Nochlin for help developing the pedigrees and H. Orr for critically reading the manuscript. This work was supported by grants from the National Ataxia Foundation, the Bob Allison Ataxia Research Center, VA research funds and the National Institutes of Health (P01 NS33718 and RO1 NS36282).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Koob, M., Moseley, M., Schut, L. et al. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). Nat Genet 21, 379–384 (1999). https://doi.org/10.1038/7710
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/7710
This article is cited by
-
A Review of Ocular Movement Abnormalities in Hereditary Cerebellar Ataxias
The Cerebellum (2023)
-
The Frequency of Intermediate Alleles in Patients with Cerebellar Phenotypes
The Cerebellum (2023)
-
Chinese abnormal compound heterozygote spinocerebellar ataxia type 8: a case report
Neurological Sciences (2022)
-
Genome sequencing broadens the range of contributing variants with clinical implications in schizophrenia
Translational Psychiatry (2021)
-
STRs: Ancient Architectures of the Genome beyond the Sequence
Journal of Molecular Neuroscience (2021)