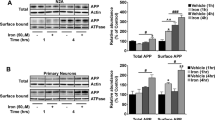
Abstract
Amyloid β protein (Aβ) deposition in the brain is a hallmark of Alzheimer's disease (AD). The fibrillar form of Aβ is neurotoxic, although the mechanism of its toxicity is unknown. We showed that conversion of Aβ to the fibrillar form markedly increased binding to specific neuronal membrane proteins, including amyloid precursor protein (APP). Nanomolar concentrations of fibrillar Aβ bound cell-surface holo-APP in cortical neurons. Reduced vulnerability of cultured APP-null neurons to Aβ neurotoxicity suggested that Aβ neurotoxicity involves APP. Thus Aβ toxicity may be mediated by the interaction of fibrillar Aβ with neuronal membrane proteins, notably APP. An Aβ–APP interaction reminiscent of the pathogenic mechanism of prions may thus contribute to neuronal degeneration in AD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Yankner, B.A. Mechanisms of neuronal degeneration in Alzheimer's disease. Neuron 16, 921–932 (1996).
Selkoe, D.J. Alzheimer's disease: genotypes, phenotypes and treatments. Science 275, 630–631 (1997).
Goate, A. et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 349, 704–706 (1991).
Citron, M. et al. Mutation of the β-amyloid precursor protein in familial Alzheimer's disease increases β-protein production. Nature 360, 672–674 (1992).
Suzuki, N. et al. An increased percentage of long amyloid β protein secreted by familial amyloid β protein precursor (βAPP717) mutants. Science 264, 1336–1340 (1994).
Scheuner, D. et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat. Med. 2, 864–870 (1996).
Yankner, B. A., Duffy, L. K. & Kirschner, D.A. Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science 250, 279–282 (1990).
Pike, C. J., Walencewicz, A. J., Glabe, C. G. & Cotman, C. W. In vitro aging of β-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 563, 311–314 (1991).
Busciglio, J., Lorenzo, A. & Yankner, B. A. Methodological variables in the assessment of β amyloid neurotoxicity. Neurobiol. Aging 13, 609–612 (1992).
Pike, C. J., Burdick, D., Walencewicz, A. J., Glabe, C. G. & Cotman, C. W. Neurodegeneration induced by β-amyloid peptides in vitro: The role of peptide assembly state. J. Neurosci. 13, 1676–1687 (1993).
Mattson, M. P., Tomaselli, K. J. & Rydel, R. E. Calcium-destabilizing and neurodegenerative effects of aggregated beta-amyloid peptide are attenuated by basic FGF. Brain Res. 621, 35–49 (1993).
Lorenzo, A. & Yankner, B. A. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc. Natl. Acad. Sci. USA 91, 12243–12247 (1994).
Howlett, D. R. et al. Aggregation state and neurotoxic properties of Alzheimer beta-amyloid peptide. Neurodegeneration 4, 23–32 (1995).
Geula, G. et al. Aging renders the brain vulnerable to amyloid β-protein neurotoxicity. Nat. Med. 4, 827–831 (1998).
Behl, C., Davis, J. B., Lesley, R. & Schubert, D. Hydrogen peroxide mediates amyloid β protein toxicity. Cell 77, 817–827 (1994).
Hensley, K. et al. A model for β-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc. Natl. Acad. Sci. USA 91, 3270–3274 (1994).
Busciglio, J., Lorenzo, A., Yeh, J. & Yankner, B. A. Beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron 14, 879–888 (1995).
Zhang, C. et al. Focal adhesion kinase expressed by nerve cell lines shows increased tyrosine phosphorylation in response to Alzheimer's A beta peptide. J. Biol. Chem. 269, 25247–25250 (1994).
Kang, J. et al. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325, 733–736 (1987).
Schubert, D., Jin, L.-W., Saitoh, T. & Cole, G. The regulation of amyloid β protein precursor secretion and its modulatory role in cell adhesion. Neuron 3, 689–694 (1989).
Chen, M. & Yankner, B. A. An antibody to β-amyloid and the amyloid precursor protein inhibits cell-substratum adhesion in many mammalian cell types. Neurosci. Lett. 125, 223–226 (1991).
Breen, K. C., Bruce, M. & Anderton, B. H. Beta amyloid precursor protein mediates neuronal cell-cell and cell-surface adhesion. J. Neurosci. Res. 28, 90–100 (1991).
Milward, E. A. et al. The amyloid protein precursor of Alzheimer's disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron 9, 129–137 (1992).
Kibbey, M. C. et al. β-amyloid precursor protein binds to the neurite-promoting IKVAV site of lamin. Proc. Natl. Acad. Sci. USA 90, 10150–10153 (1993).
Saitoh, T. et al. Secreted form of amyloid β protein precursor is involved in the growth regulation of fibroblasts. Cell 58, 615–622 (1989).
Mattson, M. P. et al. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the β-amyloid precursor protein. Neuron 10, 243–254 (1993).
Schubert, D. & Behl, C. The expression of amyloid beta protein precursor protects nerve cells from β-amyloid and glutamate toxicity and alters their interaction with the extracellular matrix. Brain Res. 629, 275–282 (1993).
May, P. C., Boggs, L. N. & Fuson, K. S. Neurotoxicity of human amylin in rat primary hippocampal cultures: similarity to Alzheimer's disease amyloid-beta neurotoxicity. J. Neurochem. 61, 2330–2333 (1993).
Lorenzo, A., Razzaboni, B., Weir, G. C. & Yankner, B. A. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature 368, 756–760 (1994).
Yan, S. D. et al. RAGE and amyloid-βpeptide neurotoxicity in Alzheimer's disease. Nature 382, 685–691 (1996).
White, A. R. et al. Survival of cultured neurons from amyloid precursor protein knock-out mice against Alzheimer's amyloid-beta toxicity and oxidative stress. J. Neurosci. 18, 6207–6217 (1998).
Zhen, H. et al. β-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell 81, 525–531 (1995).
White, A. R. et al. The Alzheimer's disease amyloid precursor protein modulates copper-induced toxicity and oxidative stress in primary neuronal cultures. J. Neurosci. 19, 9170–9179 (1999).
Yoshikawa, K., Aizawa, T. & Hayashi, Y. Degeneration in vitro of post-mitotic neurons overexpressing the Alzheimer amyloid protein precursor. Nature 359, 64–67 (1992).
Nishimura, I. et al. Degeneration in vivo of rat hippocampal neurons by wild-type Alzheimer amyloid precursor protein overexpressed by adenovirus-mediated gene transfer. J. Neurosci. 18, 2387–2398 (1998).
Bursztajn, S. et al. Overexpression in neurons of human presenilin-1 or a presenilin-1 familial Alzheimer disease mutant does not enhance apoptosis. J. Neurosci. 18, 9790–9799 (1998).
Xu, X. et al. Wild-type but not Alzheimer-mutant amyloid precursor protein confers resistance against p53-mediated apoptosis. Proc. Natl. Acad. Sci. USA 96, 7547–7552 (1999).
Uetsuki, T. et al. Activation of neuronal caspase-3 by intracellular accumulation of wild-type Alzheimer amyloid precursor protein. J. Neurosci. 19, 6955–6964 (1999).
Russo, T. et al. Fe65 and the protein network centered around the cytosolic domain of the Alzheimer's beta-amyloid precursor protein. FEBS Lett. 434, 1–7 (1998).
Yamatsuji, T. et al. G protein-mediated neuronal DNA fragmentation induced by familial Alzheimer's disease-associated mutants of APP. Science 272, 1349–1352 (1996).
Perez, R. G., Zheng, H., Van de Ploeg, L. H. T. & Koo, E. H. The β-amyloid precursor protein of Alzheimer's disease enhances neuron viability and modulates neuronal polarity. J. Neurosci. 17, 9407–9414 (1997).
Bueler H. et al. Mice devoid of PrP are resistant to scrapie. Cell 73, 1339–1347 (1993).
Brown, D. R., Schmidt, B. & Kretzschmer, H. A. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 380, 345–347 (1996).
Hegde R. S. et al. Transmissible and genetic prion diseases share a common pathway of neurodegeneration. Nature 402, 822–826 (1999).
Davis-Salinas, J., Saporito-Irwin, S. M., Cotman, C. W. & Van Nostrand W. E. Amyloid beta-protein induces its own production in cultured degenerating cerebrovascular smooth muscle cells. J. Neurochem. 65, 931–934 (1995).
Yang, A., Chandswangbhuvana, D., Shu, T., Henschen, A., & Glabe, C. G. Intracellular accumulation of insoluble, newly synthesized Aβn-42 in amyloid precursor protein-transfected cells that have been treated with Aβ1-42. J. Biol. Chem. 274, 20650–20656 (1999).
Paganetti, P. A., Lis, M., Klafki, H. -W. & Staufenbiel, M. Amyloid precursor protein truncated at any of the γ-secretase sites is not cleaved to β-amyloid. J. Neurosci. Res. 46, 283–293 (1996).
Ledermann, B. & Burki, K. Establishment of a germ-line competent C57BL/6 embryonic stem cell line. Exp. Cell Res. 197, 254–258 (1991).
Condrescu, M., Osses, L. & DiPolo, R. Partial purification and characterization of the (Ca2+, Mg2+) ATPase from squid optic nerve plasma membrane. Biochem. Biophys. Acta 769, 261–269 (1984).
Savage, M. R. et al. Turnover of amyloid β-protein in mouse brain and acute reduction of its level by phorbol ester. J. Neurosci. 18, 1743–1752 (1998).
Acknowledgements
This work was supported by NIH grants (NS30352 and NS33325), a grant from Novartis Pharma, Ltd. and a Zenith Award from the Alzheimer's Association to B.A.Y., grants from Conicet and Conicor to A.L., an NIH training grant to Z.Z. and an NIH MRRC Core grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lorenzo, A., Yuan, M., Zhang, Z. et al. Amyloid β interacts with the amyloid precursor protein: a potential toxic mechanism in Alzheimer's disease. Nat Neurosci 3, 460–464 (2000). https://doi.org/10.1038/74833
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/74833
This article is cited by
-
Rab35 and glucocorticoids regulate APP and BACE1 trafficking to modulate Aβ production
Cell Death & Disease (2021)
-
The existence of Aβ strains and their potential for driving phenotypic heterogeneity in Alzheimer’s disease
Acta Neuropathologica (2021)
-
Association of microbiota-derived propionic acid and Alzheimer’s disease; bioinformatics analysis
Journal of Diabetes & Metabolic Disorders (2020)
-
Synthesis and biological evaluation of quinoline/cinnamic acid hybrids as amyloid-beta aggregation inhibitors
Monatshefte für Chemie - Chemical Monthly (2020)
-
Synaptic and memory dysfunction induced by tau oligomers is rescued by up-regulation of the nitric oxide cascade
Molecular Neurodegeneration (2019)