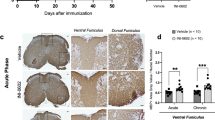
Abstract
Glutamate excitotoxicity mediated by the AMPA/kainate type of glutamate receptors damages not only neurons but also the myelin-producing cell of the central nervous system, the oligodendrocyte1. In multiple sclerosis, myelin, oligodendrocytes and some axons are lost as a result of an inflammatory attack on the central nervous system2. Because glutamate is released in large quantities by activated immune cells3, we expected that during inflammation in MS, glutamate excitotoxicity might contribute to the lesion. We addressed this by using the AMPA/kainate antagonist NBQX to treat mice sensitized for experimental autoimmune encephalomyelitis, a demyelinating model that mimics many of the clinical and pathologic features of multiple sclerosis. Treatment resulted in substantial amelioration of disease, increased oligodendrocyte survival and reduced dephosphorylation of neurofilament H, an indicator of axonal damage4. Despite the clinical differences, treatment with NBQX had no effect on lesion size and did not reduce the degree of central nervous system inflammation. In addition, NBQX did not alter the proliferative activity of antigen-primed T cells in vitro, further indicating a lack of effect on the immune system. Thus, glutamate excitotoxicity seems to be an important mechanism in autoimmune demyelination, and its prevention with AMPA/kainate antagonists may prove to be an effective therapy for multiple sclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
McDonald, J.W., Althomsons, S.P., Hyrc, K.L., Choi, D. & Goldberg, M.P. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nature Med. 4, 291–297 ( 1998).
Prineas, J.W. & McDonald, I. in Greenfield's Neuropathology (eds. Graham D.I. & Lantos P.L.) 813–881 (Arnold, New York, 1997).
Piani, D., Frei, K., Do, K.Q., Cuénod, M. & Fontana, A. Murine brain macrophages induce NMDA receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci. Lett. 133, 159–162 ( 1991).
Trapp, B.D. et al. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 338, 278–285 (1998).
Yoshioka, A., Bacskai, B. & Pleasure, D. Pathophysiology of oligodendroglial excitotoxicity. J. Neurosci. Res. 46, 427–37 (1996).
Cannella, B. et al. The neuregulin, glial growth factor 2, diminishes autoimmune demyelination and enhances remyelination in a chronic relapsing model for multiple sclerosis. Proc. Natl. Acad. Sci. USA 95, 10100–10105 (1998).
Raine, C.S. in Multiple Sclerosis Clinical and Pathogenetic Basis (eds. Raine, C.S., McFarland, H.F. & Tourtellotte, W.W.) 243–286 (Chapman & Hall, London, 1997).
Szaro, B.G., Whitnall, M.H. & Gainer, H. Phosphorylation-dependent epitopes on neurofilament proteins and neurofilament densities differ in axons in the corticospinal and primary sensory dorsal column tracts in the rat spinal cord. J. Comp. Neurol. 302, 220–235 (1990).
Rodriguez-Moreno, A., Herreras, O. & Lerma, J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron 19, 893–901 (1997).
Selmaj, K.W. & Raine, C.S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann. Neurol. 23, 339–346 (1988).
Gijbels, K., Galardy, R.E. & Steinman, L. Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. J. Clin. Invest. 94, 2177–2182 ( 1994).
Ding, M. et al. Antisense knockdown of inducible nitric oxide synthase inhibits induction of experimental autoimmune encephalomyelitis in SJL/J mice. J. Immunol. 160, 2560–2564 (1998).
Genain, C.P., Cannella, B., Hauser, S.L. & Raine, C.S. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nature Med. 5, 170– 175 (1999).
Hardin-Pouzet, H. et al. Glutamate metabolism is down-regulated in astrocytes during experimental allergic encephalomyelitis. Glia 20, 79–85 (1997).
Buryakova, A.V. & Sytinsky, I.A. Amino acid composition of cerebrospinal fluid in acute neuroinfections in children. Arch. Neurol. 32, 28–31 (1975).
Stover, J.F. et al. Neurotransmitters in cerebrospinal fluid reflect pathological activity. Eur. J. Clin. Invest. 27, 1038 –43 (1997).
Rothman, S.M. & Olney, J.W. Excitotoxicity and the NMDA receptor . Trends Neurosci. 10, 299– 302 (1987).
Collins, R.C., Dobkin, B.H. & Choi, D.W. Selective vulnerability of the brain: new insights into the pathophysiology of stroke. Ann. Intern. Med. 110, 992–1000 (1989).
O'Neill, M.J. et al. Decahydroisoquinolines: novel competitive AMPA/kainate antagonists with neuroprotective effects in global cerebral ischaemia. Neuropharmacology 37, 1211–1222 ( 1998).
Rosenberg, L.J., Teng, Y.D., Wrathall, J. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline reduces glial loss and acute white matter pathology after experimental spinal cord contusion. J. Neurosci. 19, 464–475 (1999).
Espey, M.G., Kustova, Y., Sei, Y. & Basile, A.S. Extracellular glutamate levels are chronically elevated in the brains of LP-BM5-infected mice: a mechanism of retrovirus-induced encephalopathy. J. Neurochem. 71, 2079–2087 ( 1998).
Adams R.D. & Kubik C.S., The morbid anatomy of the demyelinative diseases. Am. J. Med. 12, 510– 518 (1952).
Acknowledgements
We thank C. Weaver, F.C. Chiu and B. Cannella for discussions; P. Cobban-Bond for administrative assistance; and M. Pakingan, N. Rempel and E. Swanson for technical assistance. This work was supported in part by the National Multiple Sclerosis Society PP0693 (P.W. and D.P.), the Singer Foundation (P.W.), United States Public Health Service grants NS 08952, NS 11920 and NS 07098; the Sol Goldman Charitable Trust -NMSS RG 1001-I-9; and the Wollowick Family Foundation (C.S.R.). D.P. is a Deutsche Forschungsgemeinschaft postdoctoral fellow.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pitt, D., Werner, P. & Raine, C. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med 6, 67–70 (2000). https://doi.org/10.1038/71555
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/71555