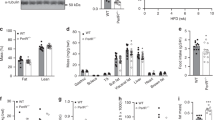
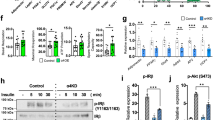
Abstract
The hallmark of type 2 diabetes, the most common metabolic disorder, is a defect in insulin–stimulated glucose transport in peripheral tissues. Although a role for phosphoinositide–3–kinase (PI3K) activity in insulin–stimulated glucose transport and glucose transporter isoform 4 (Glut4) translocation has been suggested in vitro1,2, its role in vivo and the molecular link between activation of PI3K and translocation has not yet been elucidated. To determine the role of PI3K in glucose homeostasis, we generated mice with a targeted disruption of the gene encoding the p85α regulatory subunit of PI3K (Pik3r1; refs 3, 4, 5). Pik3r1−/− mice showed increased insulin sensitivity and hypoglycaemia due to increased glucose transport in skeletal muscle and adipocytes. Insulin–stimulated PI3K activity associated with insulin receptor substrates (IRSs) was mediated via full–length p85α in wild–type mice, but via the p50α alternative splicing isoform of the same gene6,7 in Pik3r1−/− mice. This isoform switch was associated with an increase in insulin–induced generation of phosphatidylinositol(3,4,5)triphosphate (PtdIns(3,4,5)P3) in Pik3r1−/− adipocytes and facilitation of Glut4 translocation from the low–density microsome (LDM) fraction to the plasma membrane (PM). This mechanism seems to be responsible for the phenotype of Pik3r1−/− mice, namely increased glucose transport and hypoglycaemia. Our work provides the first direct evidence that PI3K and its regulatory subunit have a role in glucose homeostasis in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Carpenter, C.L. & Cantley, L.C. Phosphoinositide kinases. Curr. Opin. Cell Biol. 8, 153– 158 (1996).
Holman, G.D. & Kasaga, M. From receptor to transporter: insulin signalling to glucose transport. Diabetologia 40, 991–1003 (1997).
Escobedo, J.A. et al. cDNA cloning of a novel 85 kd protein that has SH2 domains and regulates binding of PI3–kinase to the PDGF β–receptor. Cell 65, 75–82 (1991).
Otsu, M. et al. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle–T/pp60c–src complexs, and PI3–kinase. Cell 65, 91– 104 (1991).
Skolnik, E.Y. et al. Cloning of PI3 kinase–associated p85 utilizing a novel method for expression/cloning of target proteins for receptor tyrosine kinases. Cell 65, 83–90 (1991).
Fruman, D.A., Cantley, L.C. & Carpenter, C.L. Structual organization and alternative splicing of the murine phosphoinositide 3–kinase p85α gene. Genomics 37,113–121 ( 1996).
Inukai, K. et al. p85α gene generates three isoforms of regulatory subunit for phosphatidylinositol 3–kinase (PI 3–kinase), p50α, p55α, and p85α, with different PI 3–kinase activity elevating responses to insulin. J. Biol. Chem. 272, 7873– 7882 (1997).
Carpenter, C.L. et al. Purification and characterization of phosphoinositide 3–kinase from rat liver. J. Biol. Chem. 265, 19704 –19711 (1990).
Antonetti, D.A., Algenstaedt, P. & Kahn, C.R. Insulin receptor substrate 1 binds two novel splice variants of the regulatory subunits of phosphatidylinositol 3–kinase in muscle and brain. Mol. Cell. Biol. 16, 2195–2203 (1996).
Inukai, K. et al. A novel 55–kDa regulatory subunit for phosphatidylinositol 3–kinase structually similar to p55PIK is generated by alternative splicing of the p85α gene. J. Biol. Chem. 271, 5317–5320 (1996).
Pons, S. et al. The structure and function of p55PIK reveal a new regulatory subunit for phosphatidylinositol 3–kinase. Mol. Cell. Biol. 15, 4453–4465 (1995).
Sun, X.J. et al. The structure of the insulin receptor substrate IRS–1 defines a unique signal transduction protein. Nature 352, 73–77 (1991).
Sun, X.J. et al. Role of IRS–2 in insulin and cytokine signalling. Nature 377, 173–177 ( 1995).
Kadowaki, T. et al. Signal transduction mechanism of insulin and insulin–like growth factor–1. Endocrine J. 43, S33–41 (1996).
Lavan, B.E., Lane, W.S. & Lienhard, G.E. The 60–kDa phosphotyrosine protein in insulin–treated adipocytes is a new member of the insulin receptor substrate family. J. Biol. Chem. 272, 11439–11443 (1997).
Toker, A. & Cantley, L.C. Signalling through the lipid products of phosphoinositide–3–OH kinase. Nature 387, 673–676 (1997).
Harano, Y. et al. Clinical significance of altered insulin sensitivity in diabetes mellitus assessed by glucose, insulin, and somatostatin infusion. J. Clin. Endocrinol. Metab. 52, 982– 987 (1981).
Satoh, S. et al. Use of bismannose photolabel to elucidate insulin–regulated GLUT4 subcellular trafficking kinetics in rat adipose cells. J. Biol. Chem. 268, 17820–17829 (1993).
Nave, B.T., Haigh, R.J., Hayward, A.C., Siddle, K. & Shepherd, P.R. Compartment–specific regulation of phosphoinositide 3–kinase by platelet–derived growth factor and insulin in 3T3–L1 adipocytes. Biochem. J. 318, 55–60 (1996).
Ricort, J.–M., Tanti, J.–F., Van Obberghen, E. & Le Marchand–Brustel, Y. Different effects of insulin and platelet–derived growth factor on phosphatidylinositol 3–kinase at the subcellular level in 3T3–L1 adipocytes. Eur. J. Biochem. 239, 17–22 (1996).
Yagi, T. et al. A role for Fyn tyrosine kinase in the suckling behaviour of neonatal mice. Nature 366, 742–745 (1993).
Tamemoto, H. et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate–1. Nature 372, 182–186 (1994).
Yagi, T. et al. A novel ES cell line, TT2, with high germline–differentiating potency. Anal. Biochem. 214, 70– 76 (1993).
Yamauchi, T. et al. Insulin signalling and insulin action in the muscles and livers of insulin–resistant, insulin receptor substrate–1 deficient mice. Mol. Cell. Biol. 16, 3074– 3084 (1996).
Kelly, K.L. & Ruderman, N.B. Insulin–stimulated phosphatidylinositol 3–kinase. J. Biol. Chem. 268, 4391 –4398 (1993).
Simpson, I.A. et al. Insulin–stimulated translocation of glucose transporters in the isolated rat adipose cells. Biochim. Biophys. Acta 763, 393–407 (1983).
Kaburagi, Y. et al. Role of insulin receptor substrate–1 and pp60 in the regulation of insulin–induced glucose transport and GLUT4 translocation in primary adipocytes. J. Biol. Chem. 272, 25839–25844 (1997).
Kimura, K. et al. Neurite outgrowth of PC12 cells is suppressed by wortmannin, a specific inhibitor of phosphatidylinositol 3–kinase. J. Biol. Chem. 269, 18961–18967 (1994).
Acknowledgements
We thank T. Katada, O. Hazeki, B.B. Kahn and E.U. Frevert for helpful discussion; N. Takeda for microinjection of ES cells; and S.W. Cushman for anti–Glut4 antibody. This work was supported by a Grant–in–Aid for Creative Basic Research (10NP0201) from the Ministry of Education, Science, Sports, and Culture of Japan, and by a grant from Uehara Memorial Foundation (T.K.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Terauchi, Y., Tsuji, Y., Satoh, S. et al. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85α subunit of phosphoinositide 3–kinase. Nat Genet 21, 230–235 (1999). https://doi.org/10.1038/6023
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/6023