Abstract
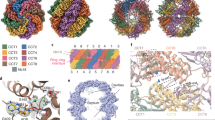
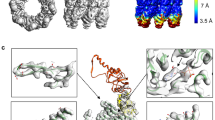
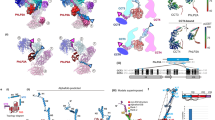
Chaperonins assist the folding of other proteins1. Type II chaperonins, such as chaperonin containing TCP–1(CCT), are found in archaea and in the eukaryotic cytosol2. They are hexadecameric or nonadecameric oligomers composed of one to eight different polypeptides. Whereas type I chaperonins like GroEL are promiscuous, assisting in the folding of many other proteins1, only a small number of proteins, mainly actin and tubulin, have been described as natural substrates of CCT. This specificity may be related to the divergence of the eight CCT subunits3. Here we have obtained a three-dimensional reconstruction of the complex between CCT and α-actin by cryo-electron microscopy and image processing. This shows that α-actin interacts with the apical domains of either of two CCT subunits. Immunolabelling of CCT–substrate complexes with antibodies against two specific CCT subunits showed that actin binds to CCT using two specific and distinct interactions: the small domain of actin binds to CCTδ and the large domain to CCTβ or CCTε (both in position 1,4 with respect to δ). These results indicate that the binding of actin to CCT is both subunit-specific and geometry-dependent. Thus, the substrate recognition mechanism of eukaryotic CCT may differ from that of prokaryotic GroEL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bukau,B. & Horwich,A. L. The hsp70 and hsp60 chaperone machines. Cell 92, 351–366 (1998).
Kubota,H., Hynes,G. & Willison,K. R. The chaperonin containing t-complex polypeptide 1 (TCP-1). Multisubunit machinery assisting in protein folding and assembly in the eukaryotic cytosol. Eur. J. Biochem. 230, 3–16 (1995).
Willison,K. R. in Molecular Chaperones and Folding Catalysts (ed. Bukau, B.) 555–571 (Harwood Academic, Amsterdam, 1999).
Braig,K. et al. The crystal structure of the bacterial chaperonin GroEL at 2.8 Å. Nature 371, 578–586 (1994).
Ditzel,L. et al. Crystal structure of the thermosome, the archaeal chaperonin and homologue of CCT. Cell 93, 125–138 (1998).
Klumpp,M., Baumeister,W. & Essen,L. O. Structure of the substrate binding domain of the thermosome, an archaeal group II chaperonin. Cell 91, 263–270 (1997).
Melki,R. & Cowan,N. J. Facilitated folding of actins and tubulins occurs via a nucleotide-dependent interaction between cytoplasmic chaperonin and distinctive folding intermediates. Mol. Cell. Biol. 14, 2895–2904 (1994).
Rommelaere,H., De Neve,M., Melki,R., Vandekerckhove,J. & Ampe,C. The cytosolic class II chaperonin CCT recognizes delineated hydrophobic sequences in its target proteins. Biochemistry 38, 3246–3257 (1999).
Llorca,O. et al. 3D reconstruction of the ATP-bound form of CCT reveals the asymmetric folding conformation of a type II chaperonin. Nature Struct. Biol. 6, 639–642 (1999).
Kabsch,W., Mannherz,H. G., Suck,D., Pai,E. F. & Holmes,K. C. Atomic structure of the actin:DNase I complex. Nature 347, 37–44 (1990).
Vinh,D. B.-N. & Drubin,D. G. A yeast TCP-1 like protein is required for actin function in vivo. Proc. Natl Acad. Sci. USA 91, 9116–9120 (1994).
Liou,A. K. F. & Willison,K. R. Elucidation of the subunit orientation in CCT (chaperonin containing TCP1) from the subunit composition of micro-complexes. EMBO J. 16, 4311–4316 (1997).
Kashuba,E., Pokrovskaja,K., Klein,G. & Szekely,L. Epstein-Barr virus-encoded nuclear protein EBNA-3 interacts with the ε-subunit of the t-complex protein 1 chaperonin complex. J. Hum. Virol. 2, 33–37 (1999).
Willison,K. R. et al. The t complex polyptide 1 (TCP-1) is associated with the cytoplasmic aspect of Golgi membranes. Cell 57, 621–632 (1989).
Hynes,G., Kubota,H. & Willison,K. R. Antibody characterization of two distinct conformations of the chaperonin containing TCP-1 from mouse testis. FEBS Lett. 358, 129–132 (1995).
Llorca,O. et al. ATP binding induces large conformational changes in the apical and equatorial domains of the eukaryotic chaperonin containing TCP-1 complex. J. Biol. Chem. 273, 10091–10094 (1998).
Marabini,R. & Carazo,J. M. Pattern recognition and classification of images of biological macromolecules using artificial neural networks. Biophys. J. 66, 1804–1814 (1994).
Frank,J. et al. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 116, 190–199 (1996).
Marabini,R., Herman,G. T. & Carazo,J. M. 3D reconstruction in electron microscopy using ART with smooth spherically symmetric volume elements (blobs). Ultramicroscopy 72, 53–65 (1998).
Acknowledgements
This work was partially supported by grants from the DGICYT (J.M.V. and J.L.C.). O.L. is a fellow the Comunidad Autónoma de Madrid. The UK laboratory is supported by the Cancer Research Campaign.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
Supplementary Information (PDF 791 kb)
Rights and permissions
About this article
Cite this article
Llorca, O., McCormack, E., Hynes, G. et al. Eukaryotic type II chaperonin CCT interacts with actin through specific subunits. Nature 402, 693–696 (1999). https://doi.org/10.1038/45294
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/45294