Abstract
There is a marked variation in whether people retain sufficient cognitive function to maintain their quality of life and independence in old age, even among those without dementia, so it would be valuable to identify the determinants of normal age-related cognitive change1,2. We have retested non-demented 80-year-olds who were participants in the Scottish Mental Survey of 1932, and find that the variation in their non-pathological cognitive change from age 11 to 80 is related to their apolipoprotein E (APOE) genotype. This effect of the APOE ɛ4 allele on normal cognitive ageing may be mediated by a mechanism that is at least partly independent of its predisposing effect towards Alzheimer's disease.
Similar content being viewed by others
Main
The Scottish Mental Survey of 1932 tested cognitive ability in almost everyone attending school in Scotland in June 1932 and who was born in 1921 (n = 87,498; ref. 3). From the Lothian birth cohort of 1921, 491 surviving participants (206 men) of the 1932 survey had their Moray House Test (MHT) score at age 11 traced. All lived independently in the community. Disease history and current medication were established at interview.
Subjects with Mini-Mental State Examination (MMSE) scores that were suggestive of dementia (score <24; 4 men, 4 women), or with any history of dementia-related illness (3 men, 2 women), were omitted from the study. There were 466 subjects (190 men) for whom we had Moray House Test scores at both age 11 and age 80, and whose APOE genotype we were able to determine.
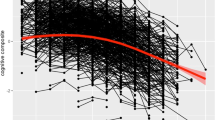
The MHT takes 45 minutes and comprises 71 verbal and non-verbal reasoning questions. It has criterion validity in youth and old age4. Up to 50% of the variation between subjects in the MHT remains stable from age 11 to about 80 (ref. 4). Subjects took the test in 1932 and again in 1999–2001. Scores at each age were controlled for age (in days) and were converted to IQ-type scores (mean, 100; s.d., 15). Subjects were classified according to the presence (n = 121) or absence (n = 345) of one or more APOE ɛ4 allele(s)5.
At age 11, MHT scores (mean, s.d.) were similar (t = 0.91, P = 0.36) for those with the ɛ4 allele (99.4, 15.2) and for those without it (100.8, 14.4). But at age about 80, MHT scores were significantly different (t = 2.64, P = 0.009) between the two groups (with the ɛ4 allele, 97.0, 15.7; without the ɛ4 allele, 101.1, 14.2).
We used analysis of covariance to examine the significance and effect sizes of contributors to cognitive change from age 11 to 80. Effects of age-11 MHT score (F1,461 = 334.8, P < 0.001, η2 = 0.42; Pearson's correlation between MHT at age 11 and at age 80, 0.65, P < 0.001), sex (F1,461 = 8.7, P = 0.003, η2 = 0.018) and presence of the ɛ4 allele (F1,461 = 5.2, P = 0.02, η2 = 0.011) were significant; sex and ɛ4 status did not interact. Cardiovascular disease history was not significantly associated with cognitive function. People with and without the APOE ɛ4 allele did not differ in the number and type of medications that they took.
We reran these models to investigate the possibility that the effects of ɛ4 might be caused by incipient dementia in some APOE ɛ4+ individuals. Including only those subjects with MMSE scores of ≤28 resulted in an increased effect of ɛ4 (F1,342 = 8.4, P = 0.004, η2 = 0.024). The effect was also evident after excluding those subjects whose IQ decrement from age 11 to 80 was greater than two standard deviations (F1,459 = 5.1, P = 0.02, η2 = 0.011).
This study has the advantage of examining the same individuals with the same cognitive test in childhood and in old age. Individual differences in childhood IQ are the most reliable predictor of late-life cognitive performance (accounting for at least 42% of variance). Possession of APOE ɛ4 is unrelated to differences in mental ability in youth, but is significantly associated with mental ability in old age and the change in ability score from youth.
Incipient dementia may have been more common in our APOE ɛ4-positive individuals and so could account for part of the effect we have observed. But we contend that this is not the only possible explanation, because the effect of APOE ɛ4 increases when the MMSE threshold is raised to include only near-perfect scorers, and remains similar after excluding those whose IQ decrement from age 11 to 80 was greater than 2 s.d. Moreover, the number of incipient cases expected in this age group6,7, and the effect size of APOE on Alzheimer's disease8, mean that each case would require a massive cognitive decline to account for the observed effects, and no such skewing or bimodal distribution was seen in either ɛ4 subgroup. The results do not seem to be due to selective attrition, because the proportion of people with ɛ4 alleles is similar to that in other, younger groups from Scotland9.
Further clinical and pathological investigation is required to determine whether Alzheimer's disease or other changes could be responsible for the differences we observe. APOE isoforms show differences in their binding to receptors, lipoproteins and amyloid-β, in atherosclerosis, neurite extension, neuroprotection and repair10. These various interactions could influence cognitive ageing independently of those that operate in Alzheimer's disease, which remain uncertain10.
Identifying a factor that influences non-pathological, lifetime cognitive change has large public-health implications because, in the absence of a sharp risk threshold, most adverse events — and most of the personal and economic burdens that these bring — occur to old people who are within the 'normal' part of the distribution11.
References
US National Research Council The Aging Mind (Natl Acad. Press, Washington DC, 2000).
Anstey, K. & Christensen, H. Gerontology 46, 163–177 (2000).
Scottish Council for Research in Education The Intelligence of Scottish Children: A National Survey of an Age Group (Univ. London Press, 1933).
Deary, I. J., Whalley, L. J., Lemmon, H., Crawford, J. R. & Starr, J. M. Intelligence 28, 49–55 (2000).
Wenham, P. R., Price, W. P. & Blundell, G. Lancet 33, 1158–1159 (1991).
Breitner, J. C. et al. Neurology 55, 161–162 (2000).
Corder, E. H. et al. Nature Genet. 7, 180–184 (1994).
Slooter, A. J. et al. Arch. Neurol. 55, 964–968 (1998).
Robertson, F. W. & Cumming, A. M. Arteriosclerosis 5, 283–292 (1985).
Mahley, R. W. & Rall, S. C. Jr Annu. Rev. Genom. Hum. Genet. 1, 507–537 (2000).
Rose, G. The Strategy of Preventive Medicine (Oxford Univ. Press, 1992).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Deary, I., Whiteman, M., Pattie, A. et al. Cognitive change and the APOE ɛ4 allele. Nature 418, 932 (2002). https://doi.org/10.1038/418932a
Issue Date:
DOI: https://doi.org/10.1038/418932a
This article is cited by
-
Loneliness 5 years ante-mortem is associated with disease-related differential gene expression in postmortem dorsolateral prefrontal cortex
Translational Psychiatry (2018)
-
Apolipoprotein-E (Apoe) ε4 and cognitive decline over the adult life course
Translational Psychiatry (2018)
-
Arylsulphatase A Pseudodeficiency (ARSA-PD), hypertension and chronic renal disease in Aboriginal Australians
Scientific Reports (2018)
-
In silico analyses of deleterious missense SNPs of human apolipoprotein E3
Scientific Reports (2017)
-
Variants near CHRNA3/5 and APOE have age- and sex-related effects on human lifespan
Nature Communications (2016)