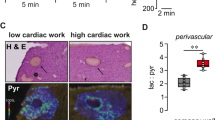
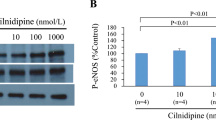
Abstract
Subcellular localization of nitric oxide (NO) synthases with effector molecules is an important regulatory mechanism for NO signalling1. In the heart, NO inhibits L-type Ca2+ channels2 but stimulates sarcoplasmic reticulum (SR) Ca2+ release3,4,5, leading to variable effects on myocardial contractility. Here we show that spatial confinement of specific NO synthase isoforms regulates this process. Endothelial NO synthase (NOS3) localizes to caveolae6,7,8, where compartmentalization with β-adrenergic receptors and L-type Ca2+ channels9 allows NO to inhibit β-adrenergic-induced inotropy8,10. Neuronal NO synthase (NOS1), however, is targeted to cardiac SR11. NO stimulation of SR Ca2+ release via the ryanodine receptor (RyR) in vitro3, 4 suggests that NOS1 has an opposite, facilitative effect on contractility. We demonstrate that NOS1-deficient mice have suppressed inotropic response, whereas NOS3-deficient mice have enhanced contractility, owing to corresponding changes in SR Ca2+ release. Both NOS1−/− and NOS3−/− mice develop age-related hypertrophy, although only NOS3−/− mice are hypertensive. NOS1/3−/− double knockout mice have suppressed β-adrenergic responses and an additive phenotype of marked ventricular remodelling. Thus, NOS1 and NOS3 mediate independent, and in some cases opposite, effects on cardiac structure and function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fang, M. et al. Dexras1: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron 28, 183–193 (2000).
Mery, P. F., Pavoine, C., Belhassen, L., Pecker, F. & Fischmeister, R. Nitric oxide regulates cardiac Ca2+ current. Involvement of cGMP-inhibited and cGMP-stimulated phosphodiesterases through guanylyl cyclase activity. J. Biol. Chem. 268, 26286–26295 (1993).
Xu, L., Eu, J. P., Meissner, G. & Stamler, J. S. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 279, 234–237 (1998).
Eu, J. P., Sun, J., Xu, L., Stamler, J. S. & Meissner, G. The skeletal muscle calcium release channel: Coupled O2 sensor and NO signaling functions. Cell 102, 499–509 (2000).
Petroff, M. G. et al. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nature Cell Biol. 3, 867–873 (2001).
Feron, O., Saldana, F., Michel, J. B. & Michel, T. The endothelial nitric-oxide synthase-caveolin regulatory cycle. J. Biol. Chem. 273, 3125–3128 (1998).
Carcia-Cardena, G. et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin: Functional significance of the NOS caveolin binding domain in vivo. J. Biol. Chem. 272, 25437–25440 (1997).
Hare, J. M. et al. Contribution of caveolin protein abundance to augmented nitric oxide signaling in conscious dogs with pacing-induced heart failure. Circ. Res. 86, 1085–1092 92000).
Schwencke, C. et al. Compartmentation of cyclic adenosine 3′,5′-monophosphate signaling in caveolae. Mol. Endocrinol. 13, 1061–1070 (1999).
Hare, J. M., Givertz, M. M., Creager, M. A. & Colucci, W. S. Increased sensitivity to nitric oxide synthase inhibition in patients with heart failure: Potentiation of β-adrenergic inotropic responsiveness. Circulation 97, 161–166 (1998).
Xu, K. Y., Huso, D. L., Dawson, T., Bredt, D. S. & Becker, L. C. NO synthase in cardiac sarcoplasmic reticulum. Proc. Natl Acad. Sci. USA 96, 657–662 (1999).
Varghese, P. et al. β(3)-adrenoceptor deficiency blocks nitric oxide-dependent inhibition of myocardial contractility. J. Clin. Invest. 106, 697–703 (2000).
Hare, J. M., Kass, D. A. & Stamler, J. S. The physiological response to cardiovascular ‘orphan’ G protein-coupled receptor agonists. Nature Med. 5, 1241–1242 (1999).
Gyurko, R., Kuhlencordt, P., Fishman, M. C. & Huang, P. L. Modulation of mouse cardiac function in vivo by eNOS and ANP. Am. J. Physiol. Heart Circ. Physiol. 278, H971–H981 (2000).
Cheng, H. J. et al. Upregulation of functional β(3)-adrenergic receptor in the failing canine myocardium. Circ. Res. 89, 599–606 (2001).
Schmidt, A. G. et al. Cardiac-specific overexpression of calsequestrin results in left ventricular hypertrophy, depressed force-frequency relation and pulsus alternans in vivo. J. Mol. Cell. Cardiol. 32, 1735–1744 (2000).
Zhai, J. et al. Cardiac-specific overexpression of a superinhibitory pentameric phospholamban mutant enhances inhibition of cardiac function in vivo. J. Biol. Chem. 275, 10538–10544 (2000).
Molkentin, J. D. et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93, 215–228 (1998).
Yang, X. P. et al. Endothelial nitric oxide gene knockout mice: cardiac phenotypes and the effect of angiotensin-converting enzyme inhibitor on myocardial ischemia/reperfusion injury. Hypertension 34, 24–30 (1999).
Verdecchia, P. et al. Adverse prognostic significance of concentric remodeling of the left ventricle in hypertensive patients with normal left ventricular mass. J. Am. Coll. Cardiol. 1925, 871–878 (1995).
Topol, E. J., Traill, T. A. & Fortuin, N. J. Hypertensive hypertrophic cardiomyopathy of the elderly. N. Engl. J. Med. 312, 277–283 (1985).
Kanai, A. J. et al. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc. Natl Acad. Sci. USA 98, 14126–14131 (2001).
Kobzik, L., Reid, M. B., Bredt, D. S. & Stamler, J. S. Nitric oxide in skeletal muscle. Nature 372, 546–548 (1994).
Brenman, J. E. et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α1-syntrophin mediated by PDZ domains. Cell 84, 757–767 (1996).
Brenman, J. E., Chao, D. S., Xia, H., Aldape, K. & Bredt, D. S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 82, 743–752 (1995).
Liu, L. et al. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410, 490–494 (2001).
Stamler, J. S., Lamas, S. & Fang, F. C. Nitrosylation: the prototypic redox-based signaling mechanism. Cell 106, 675–683 (2001).
Moncada, S. A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 329, 2002–2012 (1993).
Son, H. et al. Long-term potentiation is reduced in mice that are doubly mutant in endothelial and neuronal nitric oxide synthase. Cell 87, 1015–1023 (1996).
Acknowledgements
This work was supported by grants from the NIH, the American Heart Association, and the American Federation for Aging Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests
Supplementary information
Rights and permissions
About this article
Cite this article
Barouch, L., Harrison, R., Skaf, M. et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 416, 337–339 (2002). https://doi.org/10.1038/416337a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/416337a
This article is cited by
-
S-nitrosylation of CSF1 receptor increases the efficacy of CSF1R blockage against prostate cancer
Cell Death & Disease (2022)
-
Quantitative aspects of nitric oxide production in the heart
Molecular Biology Reports (2022)
-
Neuronal nitric oxide synthase regulation of calcium cycling in ventricular cardiomyocytes is independent of Cav1.2 channel modulation under basal conditions
Pflügers Archiv - European Journal of Physiology (2020)
-
RETRACTED ARTICLE: Fibrauretine reduces ischemia/reperfusion injury via RISK/eNOS activation
Naunyn-Schmiedeberg's Archives of Pharmacology (2020)
-
Nitric oxide signalling in cardiovascular health and disease
Nature Reviews Cardiology (2018)