Abstract
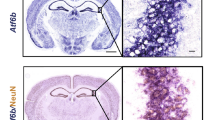
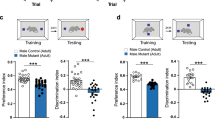
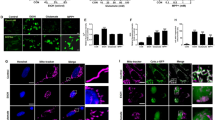
Excitatory amino acids induce both acute membrane depolarization and latent cellular toxicity, which often leads to apoptosis in many neurological disorders1,2. Recent studies indicate that glutamate toxicity may involve the c-Jun amino-terminal kinase (JNK) group of mitogen-activated protein (MAP) kinases3,4,5. One member of the JNK family, Jnk3, may be required for stress-induced neuronal apoptosis, as it is selectively expressed in the nervous system6,7. Here we report that disruption of the gene encoding Jnk3 in mice caused the mice to be resistant to the excitotoxic glutamate-receptor agonist kainic acid: they showed a reduction in seizure activity and hippocampal neuron apoptosis was prevented. Although application of kainic acid imposed the same level of noxious stress, the phosphorylation of c-Jun and the transcriptional activity of the AP-1 transcription factor complex were markedly reduced in the mutant mice. These data indicate that the observed neuroprotection is due to the extinction of a Jnk3-mediated signalling pathway, which is animportant component in the pathogenesis of glutamate neurotoxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Choi, D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1, 623–634 (1988).
Coyle, J. T. & Puttfarcken, P. Oxidative stress, glutmate, and neurodegenerative disorders. Science 262, 689–695 (1993).
Schwarzschild, M. A., Cole, R. L. & Hyman, S. E. Glutamate, but not dopamine, stimulates stress-activated protein kinase and AP-1-mediated transcription in striatal neurons. J. Neurosci. 17, 3455–3466 (1997).
Kawasaki, H. et al. Activation and involvement of p38 mitogen-activated protein kinase in glutamate-induced apoptosis in rat cerebellar granule cells. J. Biol. Chem. 272, 18518–18521 (1997).
Xia, Z., Dickens, M., Raingeaud, J., Davis, R. J. & Greenberg, M. E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270, 1326–1331 (1995).
Martin, J. H., Mohit, A. A. & Miller, C. A. Developmental expression in the mouse nervous system of the p493F12 SAP kinase. Brain Res. Mol. Brain Res. 35, 47–57 (1996).
Gupta, S. et al. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 15, 2760–2770 (1996).
Kyriakis, J. M. et al. The stress-activated protein kinase subfamily of c-Jun kinases. Nature 369, 156–160 (1994).
Derijard, B. et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76, 1025–1037 (1994).
Whitmarsh, A. J. & Davis, R. J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74, 589–607 (1996).
Derijard, B. et al. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267, 682–685 (1995).
Nishina, H. et al. Stress-signalling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature 385, 350–353 (1997).
Sanchez, I. et al. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature 372, 794–798 (1994).
Yang, D. et al. Targeted disruption of the MKK4 gene causes embryonic death, inhibition of JNK activation and defects in AP-1 transcriptional activity. Proc. Natl Acad. Sci. USA 94, 3004–3009 (1997).
Tournier, C., Whitmarsh, A. J., Cavanagh, J., Barrett, T. & Davis, R. J. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc. Natl Acad. Sci. USA 94, 7337–7342 (1997).
Gupta, S., Campbell, D., Derijard, B. & Davis, R. J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science 267, 389–393 (1995).
Ben-Ari, Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 14, 375–403 (1985).
Ferkany, J. W., Zaczek, R. & Coyle, J. T. The mechanism of kainic acid neurotoxicity. Nature 308, 561–562 (1984).
Morgan, J. I. & Curran, T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu. Rev. Neurosci. 14, 421–451 (1991).
Kasof, G. M. et al. Kanic acid-induced neuronal death is associated with DNA damage and a unique immediate-early gene response in c-fos-lacZ transgenic rats. J. Neurosci. 15, 4238–4249 (1995).
Morgan, J. I., Cohen, D. R., Hempstead, J. L. & Curran, T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science 237, 192–197 (1987).
Berger, M. & Ben-Ari, Y. Autoradiographic visualization of [3H]kainic acid receptor subtypes in the rat hippocampus. Neurosci. Lett. 39, 237–242 (1983).
Westbrook, G. L. & Lothman, E. W. Cellular and synaptic basis of kainic acid-induced hippocampal epileptiform activity. Brain Res. 273, 97–109 (1983).
Rincn, M. & Flavell, R. A. AP-1 transcriptional activity requires both T-cell receptor-mediated and co-stimulatory signals in primary T lymphocytes. EMBO J. 13, 4370–4381 (1994).
Rothman, S. M., Thurston, J. H. & Hauhart, R. E. Delayed neurotoxicity of excitatory amino acids in vitro. Neuroscience 22, 471–480 (1987).
Bonfoco, E., Krainc, D., Ankarcrona, M., Nicotera, P. & Lipton, S. A. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc. Natl Acad. Sci. USA 92, 7162–7166 (1995).
Mohit, A. A., Martin, J. H. & Miller, C. A. p493F12 kinase: a novel MAP kinase expressed in a subset of neurons in the human nervous system. Neuron 14, 67–78 (1995).
Lipton, S. A. & Rosenberg, P. A. Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 330, 613–622 (1994).
Rothman, S. M. & Olney, J. W. Glutamate and the pathophysiology of hypoxic-ischemic brain damage. Ann. Neurol. 19, 105–111 (1986).
Tsirka, S. E., Gualandris, A., Amaral, D. G. & Strickland, S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature 377, 340–344 (1995).
Acknowledgements
This Letter is dedicated to the memory of Morton Nathanson, who suggested key experiments. We thank S. Benzer, D. Y. Loh, C. A. Miller, J. I. Morgan and C. L. Stewart for providing reagents; and T. Barratt, J. Bao, D. Butkis, L. Evangelisti, C. Hughes and J. Musco for technical assistance. R.J.D. and R.A.F. are investigators, and D.D.Y. is an associate, of the Howard Hughes Medical Institute. This work was supported by the Howard Hughes Medical Institute (R.J.D. and R.A.F.) and Public Health Service grants (R.J.D. and P.R.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, D., Kuan, CY., Whitmarsh, A. et al. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 389, 865–870 (1997). https://doi.org/10.1038/39899
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/39899