Abstract
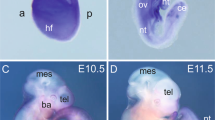
The arylhydrocarbon-receptor nuclear translocator (ARNT) is a member of the basic-helix-loop-helix–PAS family of heterodimeric transcription factors which includes the arylhydrocarbon receptor (AHR), hypoxia-inducible factor-1α (HIF-1α) and the Drosophila single-minded protein (Sim)1–4. ARNT forms heterodimeric complexes with the arylhydrocarbon receptor, HIF-1α, Sim and the PAS protein Per2,4–6. In response to environmental pollutants, AHR–ARNT heterodimers regulate genes involved in the metabolism of xenobiotics7–9, whereas ARNT–HIF-1α heterodimers probably regulate those involved in the response to oxygen deprivation10–13. By generating a targeted disruption of the Arnt locus in the mouse, we show here that Arnt–/– embryonic stem cells fail to activate genes that normally respond to low oxygen tension. Arnt–/– ES cells also failed to respond to a decrease in glucose concentration, indicating that ARNT is crucial in the response to hypoxia and to hypoglycaemia. Arnt–/– embryos were not viable past embryonic day 10.5 and showed defective angiogenesis of the yolk sac and branchial arches, stunted development and embryo wasting. The defect in blood vessel formation in Arnt–/– yolk sacs is similar to the angiogenic abnormalities reported for mice deficient in vascular endothelial growth factor14,15 or tissue factor16. On the basis of these findings, we propose a model in which increasing tissue mass during organogenesis leads to the formation of hypoxic/nutrient-deprived cells, the subsequent activation of ARNT, and a concomitant increase in the expression of genes (including that encoding vascular endothelial growth factor) that promote vascularization of the developing yolk sac and solid tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Burbach, K. M., Poland, A. & Bradfield, C. A. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc. Natl Acad. Sci. USA 89, 8185–8189 (1992).
Wang, G. L., Jiang, B.-H., Rue, E. A. & Semenza, G. L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix–PAS heterodimer regulated by cellular O.2 tension. Proc. Natl Acad. Sci. USA 92, 5510–5514 (1995).
Ema, M. et al. cDNA cloning of a murine homologue of Drosophila single-mided, its mRNA expression in mouse development, and chromosome localization. Biochem. Biophys. Res. Commun. 218, 588–594 (1996).
Hoffman, E. C. et al. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science 252, 954–958 (1991).
Swanson, H. I., Chan, W. K. & Bradfield, C. A. DNA binding specificities and pairing rules of Ah receptor, ARNT, and SIM proteins. J. Biol. Chem. 270, 26292–26302 (1995).
Sogawa, K. et al. Possible function of Ah receptor nuclear translocator (Arnt) homodimer in transcriptional regulation. Proc. Natl Acad. Sci. USA 92, 1936–1940 (1995).
Nebert, D. W. & Gonzales, F. J. P450 genes: structure, evolution, and regulation. Annu. Rev. Biochem. 56, 945–993 (1987).
Telakowski-Hopkins, C., King, R. & Pickett, C. Glutathione S-transferase Ya subunit gene: identification of regulatory elements required for basal level and inducible expression. Proc. Natl Acad. Sci. USA 85, 1000–1004 (1988).
Hankinson, O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 35, 307–340 (1995).
Wang, G. L. & Semenza, G. L. General involvement of hypoxia-inducible factor in transcriptinal response to hypoxia. Proc. Natl Acad. Sci. USA 90, 4304–4308 (1993).
Forsythe, J. A. et al. Activation of vesculer endothelial growth factor gene transcription by hypoxiainducible factor 1. Mol. Cell. Biol. 16, 4604–4613 (1995).
Wang, G. L. & Semenza, G. L. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 268, 21513–21518 (1993).
Bunn, H. F. & Poyton, R. O. Oxygen sensing and molecular adaptation to hypoxia. Physiol. Rev. 76, 839–885 (1996).
Carmeliet, P. et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435–439 (1996).
Ferrara, N. et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380, 439–442 (1996).
Carmeliet, P. et al. Role of tissue factor in embryonic blood vessel development. Nature 383, 73–75 (1996).
Mortensen, R., Conner, D., Chao, S., Geisterfer-Lowrance, A. & Seidman, J. Production of homozygous mutant ES cells with a single targeting construct. Mol. Cell. Biol. 12, 2391–2394 (1992).
Shweiki, D., Itin, A., Softer, D. & Keshet, E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-intiated angiogenesis. Nature 359, 843–845 (1992).
Firth, J. D., Ebert, B. L., Pugh, C. W. & Ratcliffe, P. J. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: Similarities with the erythropoietin 3′ enhancer. Proc. Natl Acad. Sci. USA 91, 6496–6500 (1994).
Semenza, G. L., Roth, P. H., Fang, H.-M. & Wang, G. L. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269, 23757–23769 (1994).
Wood, S. M., Gleadle, J. M., Pugh, C. W., Hankinson, O. & Ratcliffe, P. J. The role of aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxia induction of gene expression. J. Biol. Chem. 271, 15117–15123 (1996).
Shweiki, D., Neeman, M., Itin, A. & Keshet, E. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: Implications for tumor angiogenesis. Proc. Natl Acad. Sci. USA 92, 768–772 (1995).
Stein, I., Neeman, M., Shweiki, D., Itin, A. & Keshet, E. Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulation with other ischemia-induced genes. Mol. Cell. Biol. 15, 5363–5368 (1995).
Hirose, K. et al. cDNA cloning and tissue-specific expresison of a novel basic helix-loop-helix/PAS factor (Arnt2) with close sequence similarity to the Aryl hydrocarbon receptor nuclear translocator (Arnt). Mol. Cell. Biol. 16, 1706–1713 (1996).
Compeau, C. G. et al. In situ ischemia and hypoxia enhance alveolar macrophage tissue factor expression. Am. J. Respir. Cell Mol. Biol. 11, 446–455 (1994).
Clauss, M. et al. Vascular permeability factor: A tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J. Exp. Med. 172, 1535–1545 (1990).
Zhang, Y. et al. Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J. Clin. Invest. 94, 1320–1327 (1994).
Wilk, R., Weizman, I. & Shilo, B.-Z. trachealess encodes a bHLH-PAS protein that is an inducer of tracheal cell fates in Drosophila. Genes Dev. 10, 93–102 (1996).
Isaac, D. D. & Andrew, D. J. Tubulogenesis in Drosophila: a requirement for the trachealess gene product. Genes Dev. 10, 103–117 (1996).
Tybulewicz, V., Crawford, C., Jackson, P., Bronson, R. & Mulligan, R. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65, 1153–1163 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maltepe, E., Schmidt, J., Baunoch, D. et al. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386, 403–407 (1997). https://doi.org/10.1038/386403a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/386403a0