Abstract
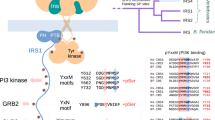
Phosphotyrosine phosphatases are critical negative or positive regulators in the intracellular signalling pathways that result in growth-factor-specific cell responses such as mitosis, differentiation, migration, survival, transformation or death1–4. The SH2-domain-containing phosphotyrosine phosphatase SHP-2 is a positive signal transducer for several receptor tyrosine kinases (RTKs) and cytokine receptors5–7. To investigate its mechanism of action we purified a tyrosine-phosphorylated glycoprotein which in different cell types associates tightly with SHP-2 and appears to serve as its substrate. Peptide sequencing in conjunction with complementary DNA cloning revealed a new gene family of at least fifteen members designated signal-regulatory proteins (SIRPs). They consist of two subtypes distinguished by the presence or absence of a cytoplasmic SHP-2-binding domain. The transmembrane polypeptide SIRPαl is a substrate of activated RTKs and in its tyrosine-phosphorylated form binds SHP-2 through SH2 interactions and acts as its substrate. It also binds SHP-1 and Grb2 in vitro and has negative regulatory effects on cellular responses induced by growth factors, oncogenes or insulin. Our findings indicate that proteins belonging to the SIRP family generally regulate signals defining different physiological and pathological processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Barford, D., Jia, Z. & Tonks, N. K. Nature Struct. Biol. 2, 1043–1053 (1995).
Traverse, S. et al. Curr. Biol. 4, 694–701 (1994).
Dikic, I., Schlessinger, J. & Lax, I. Curr. Biol 4, 702–708 (1994).
Obermeier, A., Tinhofer, I., Grunicke, H. H. & Ullrich, A. EMBO J. 15, 73–82 (1996).
Saltiel, A. R. FASEB J. 8, 1034–1040 (1994).
Matozaki, T. & Kasuga, M. Cell. Signal. 8, 13–19 (1996).
Streuli, M. Curr. Opin. Cell. Biol 8, 182–188 (1996).
Holgado-Madruga, M., Emlet, D. R., Moscatello, D. K., Godwin, A. K. & Wong, A. J. Nature 379, 560–564 (1996).
Herbst, R. et al. Cell 85, 899–909 (1996).
Stein-Gerlach, M., Kharitonenkov, A., Vogel, W., Ali, S. & Ullrich, A. J. Biol. Chem. 270, 24635–24637 (1995).
Su, L. et al. J. Biol. Chem. 271, 10385–10390 (1996).
Milarski, K. L. & Saltiel, A. R. J. Biol. Chem. 269, 21239–21243 (1994).
Yamauchi, K., Ribons, V., Saltiel, A. R. & Pessin, J. E. J. Biol. Chem. 270, 17716–17722 (1995).
Yamauchi, K. & Pessin, J. E. J. Biol. Chem. 270, 14871–14874 (1995).
Yamauchi, K., Milarski, K. L., Saltiel, A. R. & Pessin, J. E. Proc. Natl Acad. Sci. USA 92, 664–668 (1995).
Sawada, T., Milarski, K. L. & Saltiel, A. R. Biochem. Biophys. Res. Commun. 214, 737–743 (1995).
Xiao, S. et al. J. Biol. Chem. 269, 21244–21248 (1994).
Noguchi, T., Matozaki, T., Horita, K., Fujioka, Y. & Kasuga, M. Mol. Cell. Biol. 14, 6674–6682 (1994).
Zho, Z. et al. J. Biol. Chem. 270, 11765–11769 (1995).
Tang, T. L., Freeman, R. M. Jr, O'Reilly, A. M., Neel, B. G. & Sokol, S. Y. Cell 80, 473–483 (1995).
Bennett, A. M., Hausdorff, S. F., O'Reilly, A. M., Freeman, R. M. & Neel, B. G. Mol. Cell. Biol. 16, 1189–1202 (1996).
Li, W. et al. Mol. Cell. Biol 14, 509–517 (1994).
Bennett, A. M., Tang, T. L., Sugimoto, S., Walsh, C. T. & Neel, B. G. Proc. Natl Acad. Sci. USA 9, 7335–7339 (1994).
Vogel, W., Lammers, R., Huang, J. & Ullrich, A. Science 259, 1611–1614 (1994).
Chen, C. & Okayama, H. Mol. Cell. Biol 7, 2745–2752 (1987).
Fendly, B. M. et al. Cancer Res. 50, 1550–1558 (1990).
Soos, M. A. et al. Biochem. J. 235, 199–208 (1986).
Miller, A. D. & Rosman, G. J. Biotechniques 7, 980–988 (1989).
Pear, W. S., Nolan, G. P., Scott, M. L. & Baltimore, D. Proc. Natl Acad. Sci. 90, 8392–8396 (1993).
Redemann, N., Holzmann, B., Wagner, E. F., Schlessinger, J. & Ullrich, A. Mol. Cell. Biol 12, 491–498 (1992).
Fujioka, Y. et al. Mol Cell Biol 16, 6887–6899 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kharitonenkov, A., Chen, Z., Sures, I. et al. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 386, 181–186 (1997). https://doi.org/10.1038/386181a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/386181a0
This article is cited by
-
The landscape overview of CD47-based immunotherapy for hematological malignancies
Biomarker Research (2023)
-
The DNA damage response pathway regulates the expression of the immune checkpoint CD47
Communications Biology (2023)
-
Modified exosomal SIRPα variants alleviate white matter injury after intracerebral hemorrhage via microglia/macrophages
Biomaterials Research (2022)
-
Immune checkpoint modulators in cancer immunotherapy: recent advances and emerging concepts
Journal of Hematology & Oncology (2022)
-
Intrinsic S phase checkpoint enforced by an antiproliferative oncosuppressor cytokine
Cancer Gene Therapy (2022)