Abstract
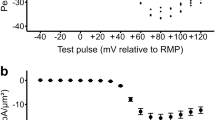
EXCITATION-CONTRACTION coupling in skeletal muscle involves a voltage sensor in the plasma membrane which, in response to depolarization, causes an intracellular calcium-release channel to open. The skeletal isoform of the ryanodine receptor (RyR-1) functions as the Ca2+-release channel1–3 and the dihydropyridine receptor (DHPR) functions as the voltage sensor and also as an L-type Ca2+ channel4,5. Here we examine the possibility that there is a retrograde signal from RyR-1 to the DHPR, using myotubes from mice homozygous for a disrupted RyR-1 gene (dyspedic mice)3. As expected, we find that there is no excitation–contraction coupling in dyspedic myotubes, but we also find that they have a roughly 30-fold reduction in L-type Ca2+-current density. Injection of dyspedic myotubes with RyR-1 complementary DNA restores excitation–contraction coupling and causes the density of L-type Ca2+ current to rise towards normal. Despite the differences in Ca2+-current magnitude, measurements of charge movement indicate that the density of DHPRs is similar in dyspedic and RyR-1-expressing myotubes. Our results support the possibility of a retrograde signal by which RyR-1 enhances the function of DHPRs as Ca2+ channels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fleischer, S. & Inui, M. A. Rev. Biophys. biophys. Chem. 18, 333–364 (1989).
McPherson, P. S. & Campbell, K. P. J. biol. Chem. 268, 13765–13768 (1993).
Takeshima, H. et al. Nature 369, 556–559 (1994).
Rios, E. & Brum, G. Nature 325, 717–720 (1987).
Tanabe, T., Beam, K. G., Powell, J. A. & Numa, S. Nature 336, 134–139 (1988).
Buck, R., Zimanyi, I., Abramson, J. J. & Pessah, I. N. J. biol. Chem. 267, 23560–23567 (1992).
Takeshima, H. et al. EMBO J. 14, 2999–3006 (1995).
Giannini, G., Conti, A., Mammarella, S., Scorbogna, M. & Sorrentino, V. J. Cell Biol. 128, 893–904 (1995).
Penner, R., Neher, E., Takeshima, H., Nishimura, S. & Numa, S. FEBS Lett. 259, 217–221 (1989).
García, J., Tanabe, T. & Beam, K. G. J. gen. Physiol. 103, 125–147 (1994).
Adams, B. A., Tanabe, T., Mikami, A., Numa, S. & Beam, K. G. Nature 346, 569–572 (1990).
Ma, J., Hosey, M. M. & Rios, E. Biophys. J. 59, 201a (1991).
Ma, J. & Coronado, R. Biophys. J. 53, 387–395 (1988).
Yatani, A. et al. J. biol. Chem. 263, 9887–9895 (1988).
Mejía-Alvarez, R., Fill, M. & Stefani, E. J. gen. Physiol. 97, 393–412 (1991).
Caffrey, M. J., Brown, M. A. & Schneider, D. M. Science 236, 570–573 (1987).
Winger, D. B. & Lansman, B. J. J. Physiol., Lond. 425, 563–578 (1990).
Dirksen, R. T. & Beam, K. G. J. gen. Physiol. 105, 227–247 (1995).
Gonzalez, A. & Rios, E. J. gen. Physiol. 102, 373–421 (1993).
Ma, J. et al. J. gen. Physiol. 102, 423–448 (1993).
Feldmeyer, D., Melzer, W., Pohl, B. & Zöllner, P. Pflügers Arch. ges. Physiol. 425, 54–61 (1993).
Takekura, H., Bennett, L., Tanabe, T., Beam, K. G. & Franzini-Armstrong, C. Biophys. J. 67, 793–803 (1994).
Takekura, H., Nishi, M., Noda, T., Takeshima, H. & Franzini-Armstrong, C. Proc. natn. Acad. Sci. U.S.A. 92, 3381–3385 (1995).
Doetschman T. C., Eistetter, H., Katz, M., Schmidt, W. & Kemler, R. J. Embryol. exp. Morph. 87, 27–45 (1985).
Gossler, A., Doetschman, T., Korn, R., Serfling, E. & Kemler, R. Proc. natn. Acad. Sci. U.S.A. 83, 9065–9069 (1986).
Takeshima, H. et al. Nature 339, 439–445 (1989).
Rando, T. A. & Blau, H. M. J. Cell Biol. 125, 1275–1287 (1994).
García, J. & Beam, K. G. J. gen. Physiol. 103, 107–123 (1994).
Adams, B. A. & Beam. K. G. J. gen. Physiol. 94, 429–444 (1989).
Tanabe, T., Adams, B. A., Numa, S. & Beam, K. G. Nature 352, 800–803 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nakai, J., Dirksen, R., Nguyen, H. et al. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature 380, 72–75 (1996). https://doi.org/10.1038/380072a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/380072a0
This article is cited by
-
Interplay between Mg2+ and Ca2+ at multiple sites of the ryanodine receptor
Nature Communications (2024)
-
Selective posttranslational inhibition of CaVβ1-associated voltage-dependent calcium channels with a functionalized nanobody
Nature Communications (2022)
-
In vivo RyR1 reduction in muscle triggers a core-like myopathy
Acta Neuropathologica Communications (2020)
-
Preclinical model systems of ryanodine receptor 1-related myopathies and malignant hyperthermia: a comprehensive scoping review of works published 1990–2019
Orphanet Journal of Rare Diseases (2020)
-
Excitation-contraction coupling in skeletal muscle: recent progress and unanswered questions
Biophysical Reviews (2020)