Abstract
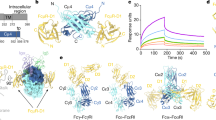
THE neonatal Fc receptor (FcRn) transports maternal immunoglobulin G (IgG) to the bloodstream of the newborn. FcRn is structurally similar to class I major histocompatibility complex (MHC) molecules1,2, despite differences in the ligands they bind (the Fc portion of IgG and antigenic peptides, respectively). A low-resolution crystal structure of the complex between FcRn and Fc localizes the binding site for Fc to the side of FcRn, distinct from the tops of the αl and α2 domains which serve as the peptide and T-cell receptor binding sites in class I molecules. FcRn binds to Fc at the interface between the Fc CH2 and CH3 domains, which contains several histidine residues that could account for the sharply pH-dependent FcRn/IgG interaction3. A dimer of FcRn heterodimers observed in the co-crystals and in the crystals of FcRn alone2 could be involved in binding Fc, correlating with the 2:1 binding stoichiometry between FcRn and IgG (ref. 4) and suggesting an unusual orientation of FcRn on the membrane.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Simister, N. E. & Mostov, K. E. Nature 337, 184–187 (1989).
Burmeister, W. P., Gastinel, L. N., Simister, N. E., Blum, M. L. & Bjorkman, P. J. Nature 372, 336–343 (1994).
Rodewald, R. J. Cell Biol. 71, 666–670 (1976).
Huber, A. H., Kelley, R. F., Gastinel, L. N. & Bjorkman, P. J. J. molec Biol. 230, 1077–1083 (1993).
Gastinel, L. N., Simister, N. E. & Bjorkman, P. J. Proc. natn. Acad. Sci. U.S.A. 89, 638–642 (1992).
Deisenhofer, J. Biochemistry 20, 2361–2370 (1981).
Harris, L. J. et al. Nature 360, 369–372 (1992).
Raghavan, M., Chen, M. Y., Gastinel, L. N. & Bjorkman, P. J. Immunity 1, 303–315 (1994).
Simister, N. E. & Mostov, K. E. Cold Spring Harbor Symp. quant. Biol. LIV, 571–580 (1989).
Salter, R. D. et al. Nature 345, 41–46 (1990).
Rodewald, R. J. Cell Biol. 58, 189–211 (1973).
Zheng, Y., Shopes, R., Holowka, D. & Baird, B. Biochemistry 30, 9125–9131 (1991).
Teng, T.-Y. J. appl. Crystallogr. 23, 387–391 (1990).
CCP4. The SERC (UK) Collaborative Computing Project No. 4, a Suite of Programs for Protein Crystallography (Daresbury Laboratory, Warrington WA4 4AD, UK, 1979).
Brünger, A. T. Acta crystallogr. A46, 46–57 (1990).
Brünger, A. T. X-PLOR (Version 3.1): A System for Crystallography and NMR (Yale University, New Haven, CT, 1992).
Guddat, L. W., Herron, J. N. & Edmundson, A. B. Proc. natn. Acad. Sci. U.S.A. 90, 4271–4275 (1993).
Otwinowski, Z. in Isomorphous Replacement and Anomalous Scattering 80–86 (Daresbury Laboratory, UK, 1991).
Leslie, A. G. W. Acta crystallogr. A43, 134–136 (1987).
Kabat, E. A., Wu, T. T., Perry, H. M., Gottesman, K. S. & Foeller, C. Sequences of Proteins of Immunological Interest (US Department of Health and Human Services, Bethesda, MD, 1991).
Wallace, K. H. & Rees, A. R. Biochem. J. 188, 9–16 (1980).
Huber, A. H. thesis, California Inst. Technol. (1994).
Hobbs, S. M., Jackson, L. E. & Peppard, J. V. J. biol. Chem. 262, 8041–8046 (1987).
Jakoi, E. R., Cambier, J. & Saslow, S. J. Immun. 135, 3360–3364 (1985).
Ahouse, J. J. et al. J. Immun. 151, 6076–6088 (1993).
Story, C. M., Mikulska, J. E. & Simister, N. E. J. exp. Med. 180, 2377–2381 (1994).
Jones, T. A., Bergdoll, M. & Kjeldgaard, M. Crystallographic Computing and Modeling Methods in Molecular Design (Springer, New York, 1993).
Kraulis, P. J. J. appl. Crystallogr. 24, 946–950 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Burmeister, W., Huber, A. & Bjorkman, P. Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature 372, 379–383 (1994). https://doi.org/10.1038/372379a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/372379a0
This article is cited by
-
The therapeutic age of the neonatal Fc receptor
Nature Reviews Immunology (2023)
-
Mechanism of antibody-specific deglycosylation and immune evasion by Streptococcal IgG-specific endoglycosidases
Nature Communications (2023)
-
Effect of posttranslational modifications and subclass on IgG activity: from immunity to immunotherapy
Nature Immunology (2023)
-
The Fab region of IgG impairs the internalization pathway of FcRn upon Fc engagement
Nature Communications (2022)
-
Biophysical differences in IgG1 Fc-based therapeutics relate to their cellular handling, interaction with FcRn and plasma half-life
Communications Biology (2022)